Deposition Date
2022-03-29
Release Date
2023-04-19
Last Version Date
2025-10-01
Entry Detail
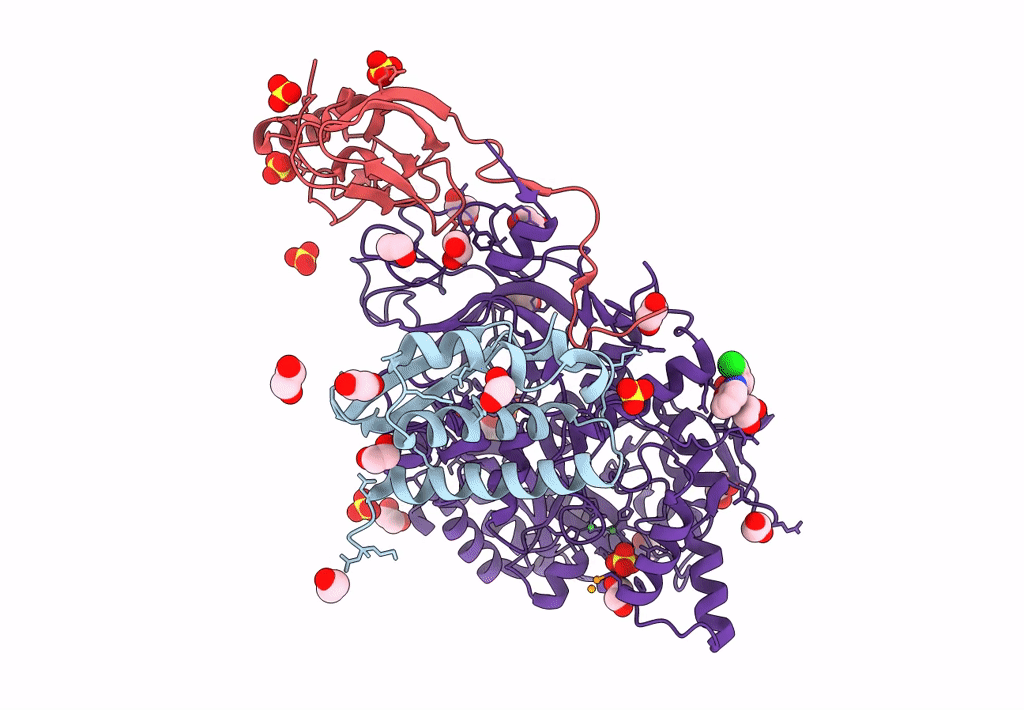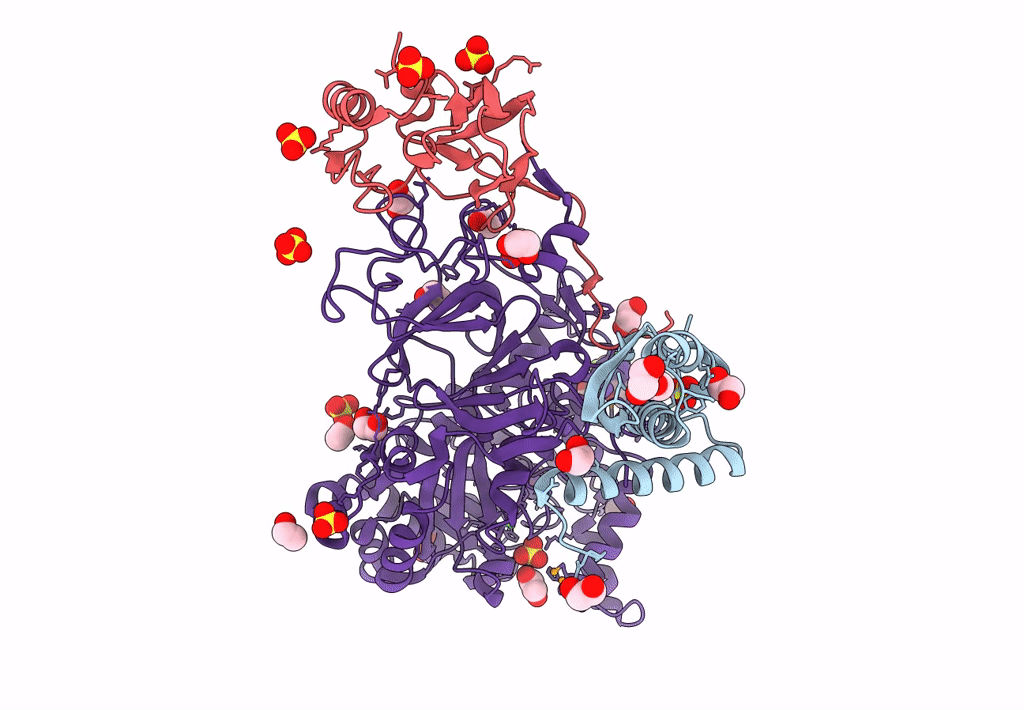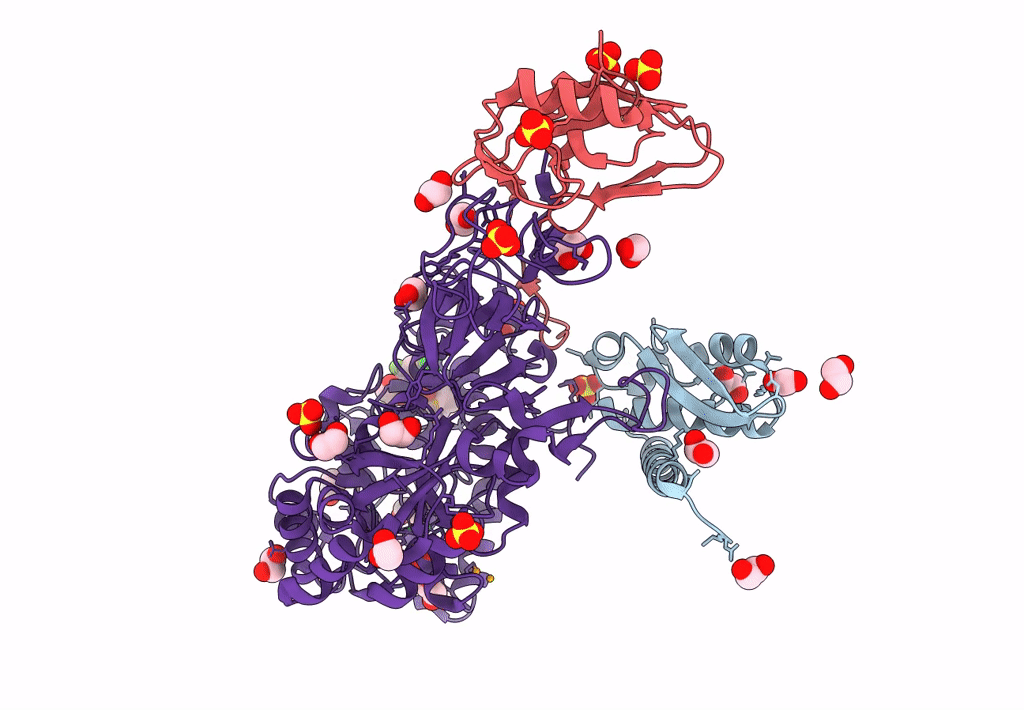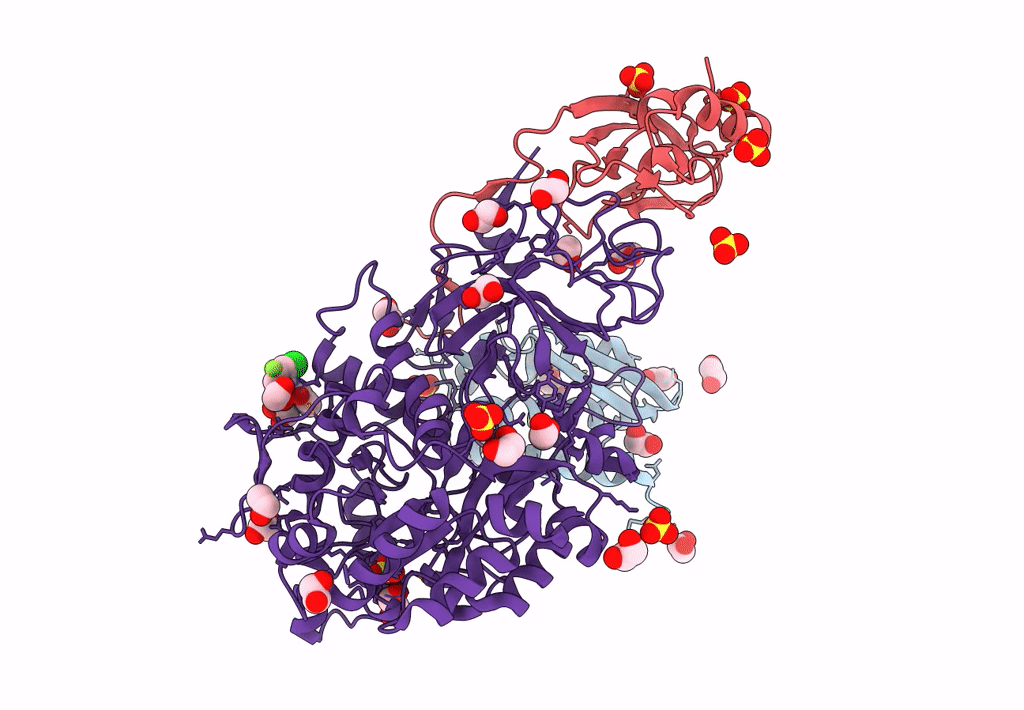
PDB ID:
7ZCY
Keywords:
Title:
Sporosarcina pasteurii urease (SPU) co-crystallized in the presence of an Ebselen-derivative and bound to Se atoms
Biological Source:
Source Organism(s):
Sporosarcina pasteurii (Taxon ID: 1474)
Method Details:
Experimental Method:
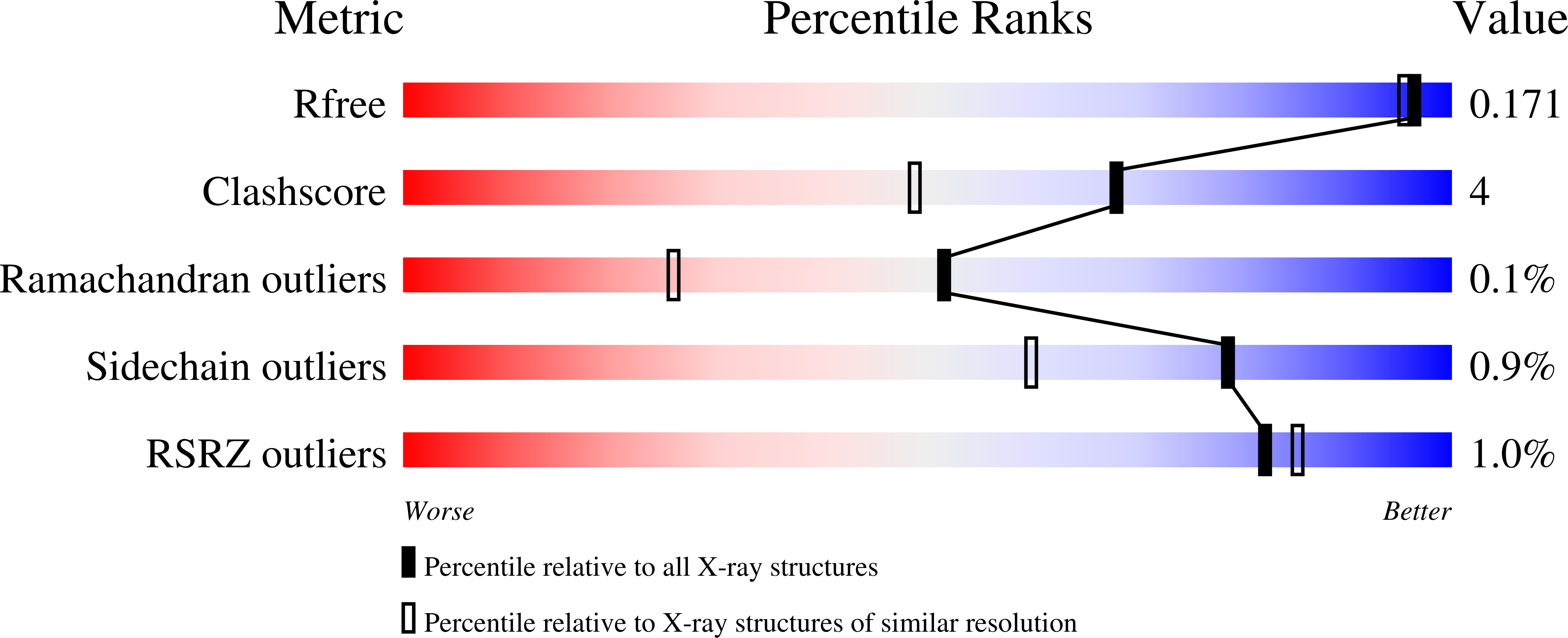
Resolution:
1.54 Å
R-Value Free:
0.15
R-Value Work:
0.13
R-Value Observed:
0.13
Space Group:
P 63 2 2