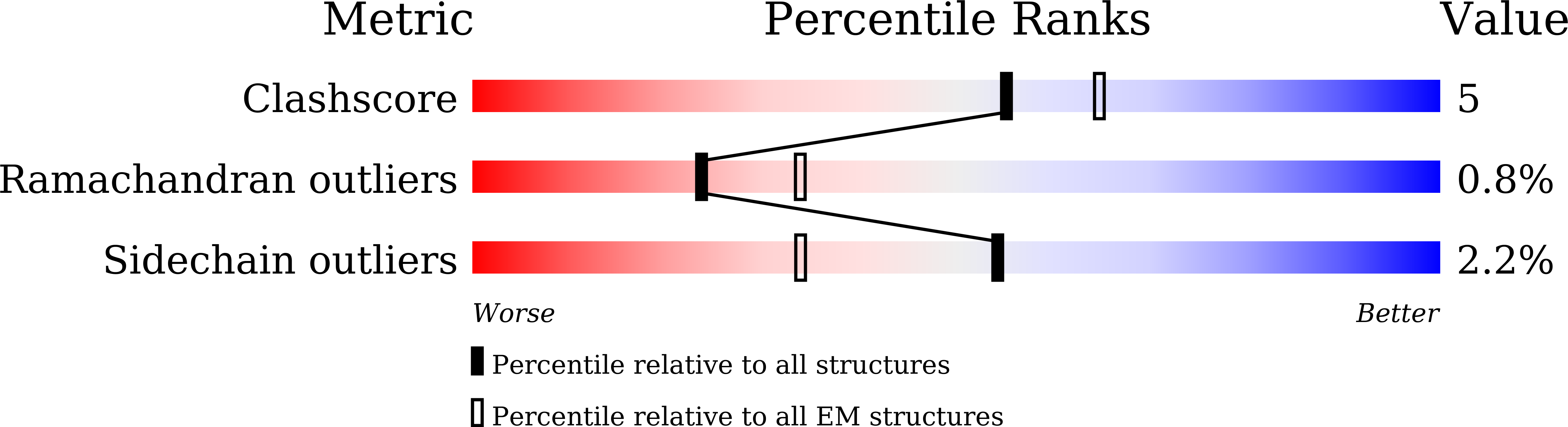
Deposition Date
2022-07-21
Release Date
2023-08-02
Last Version Date
2025-09-17
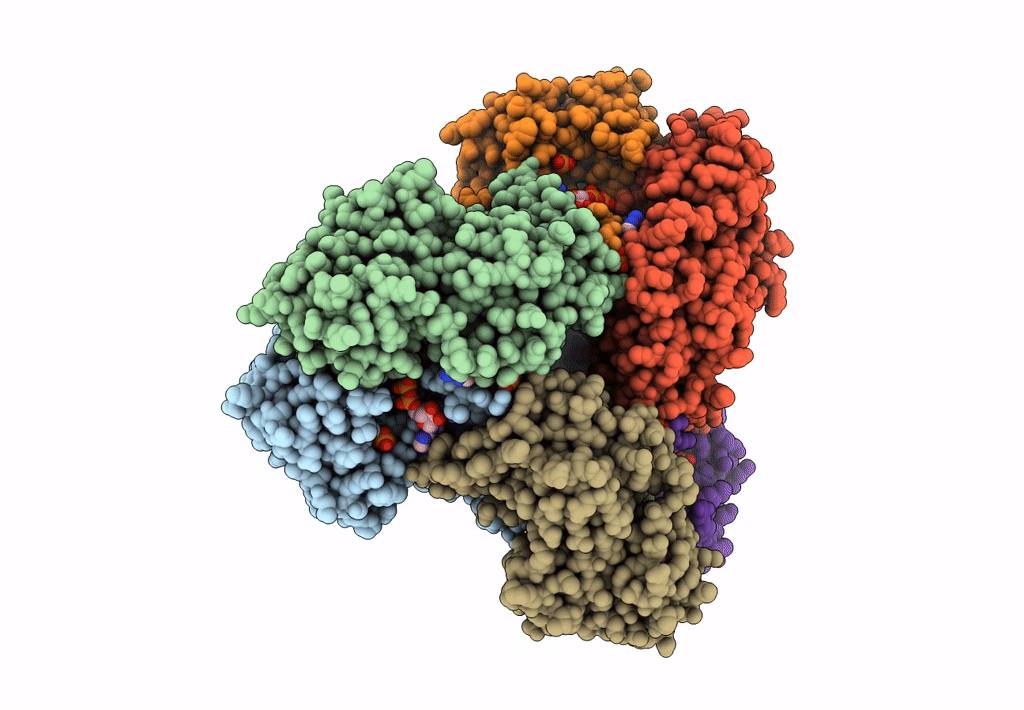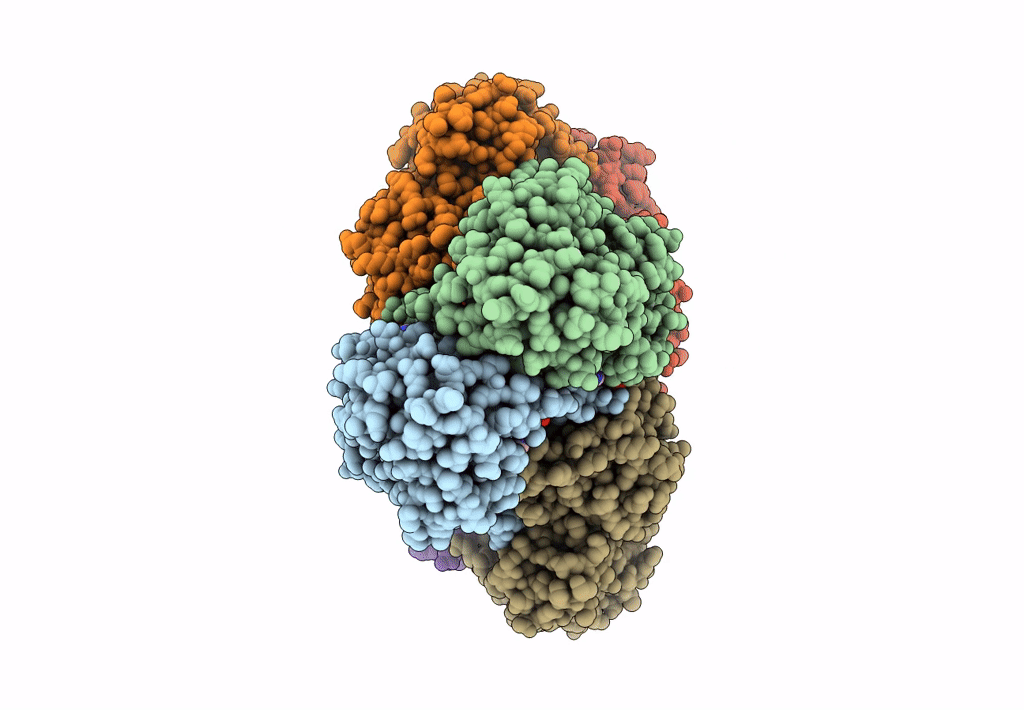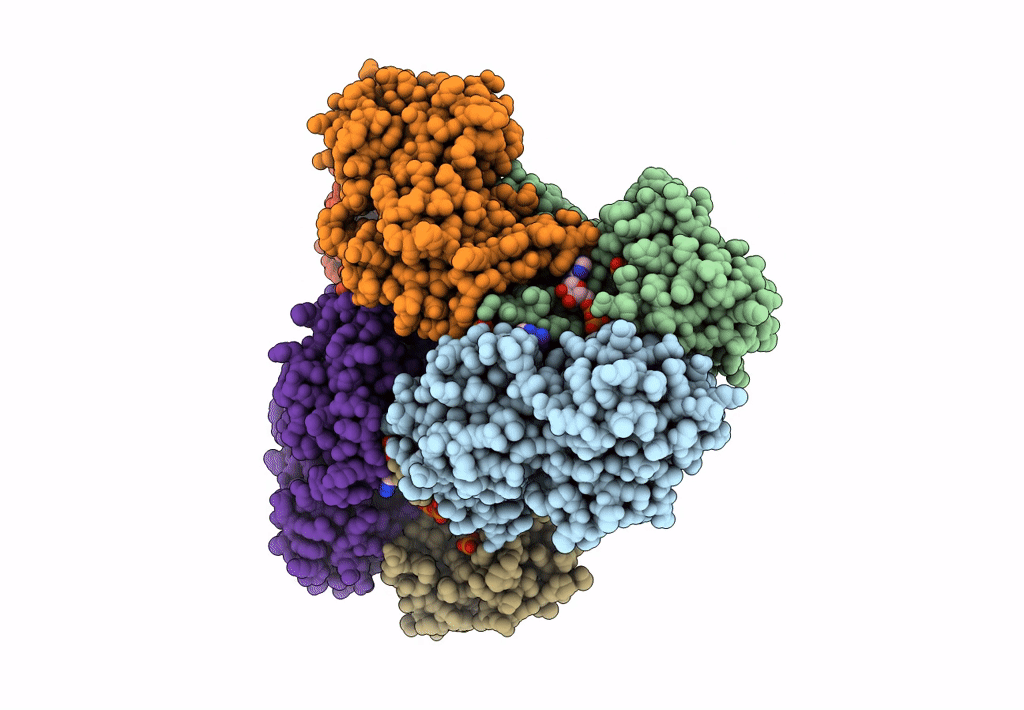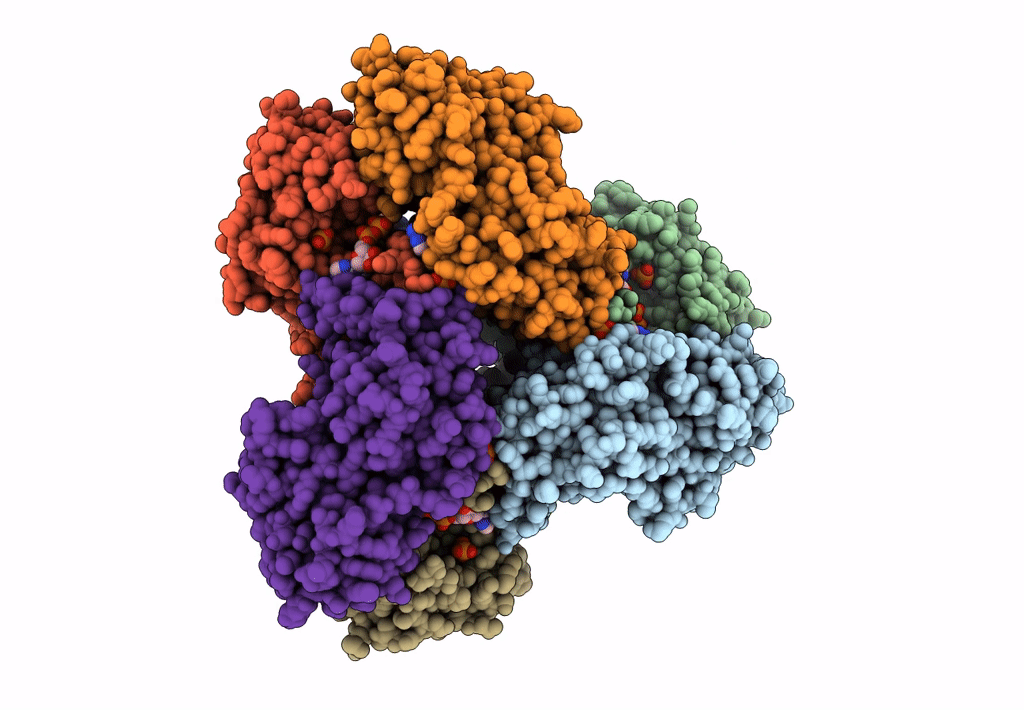
Entry Detail
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.08 Å
Aggregation State:
FILAMENT
Reconstruction Method:
SINGLE PARTICLE