Deposition Date
2022-07-13
Release Date
2023-07-19
Last Version Date
2024-11-20
Entry Detail
PDB ID:
7YHH
Keywords:
Title:
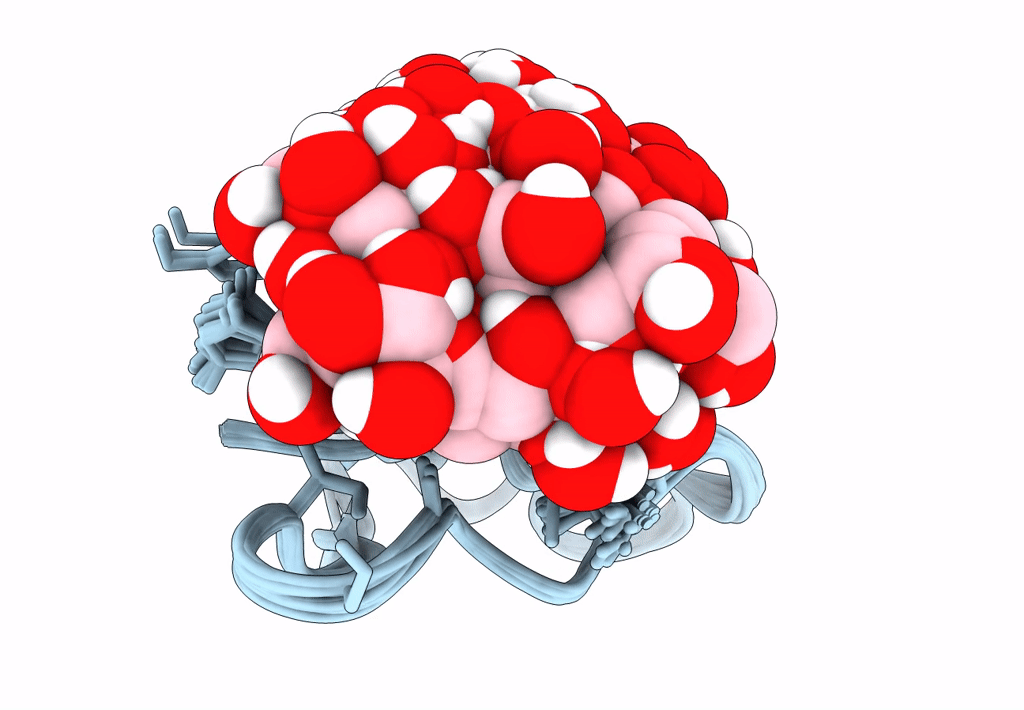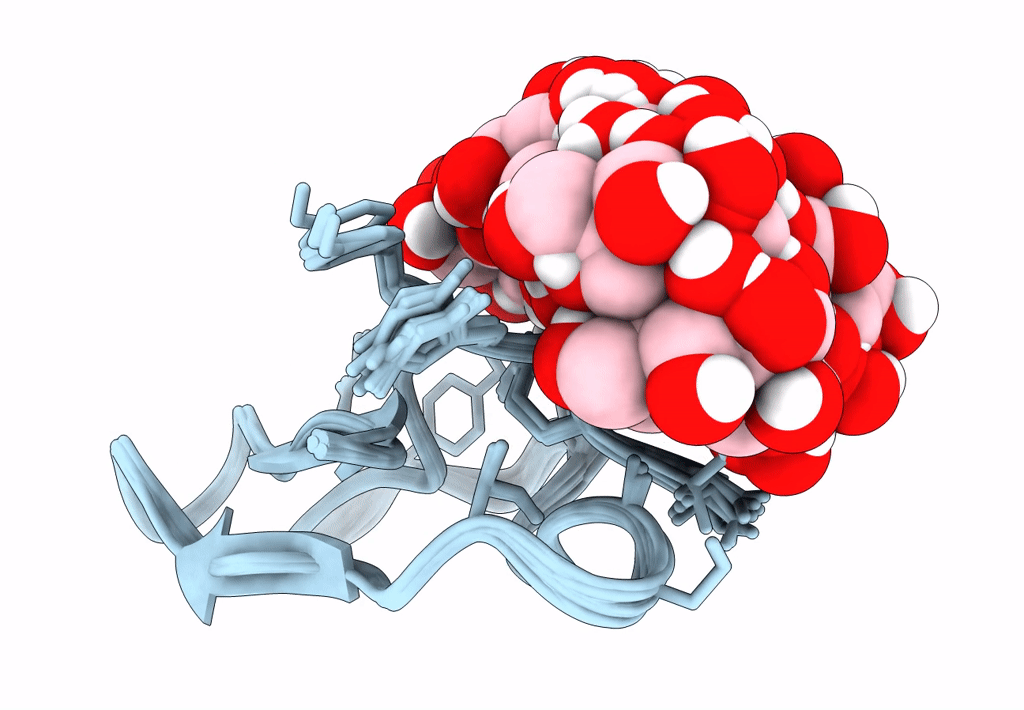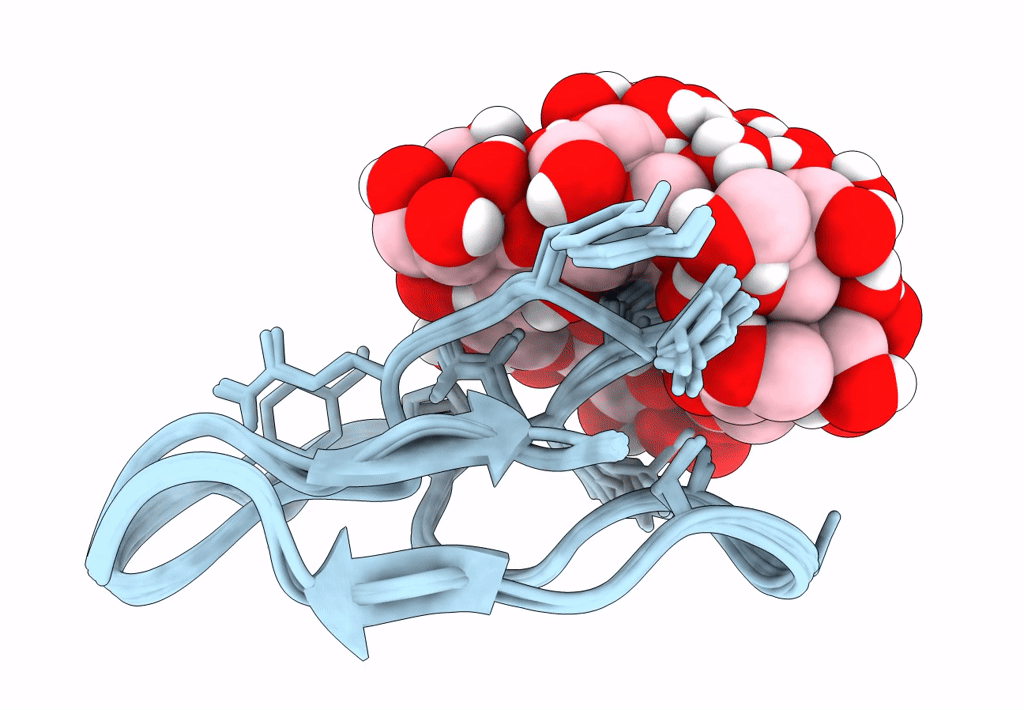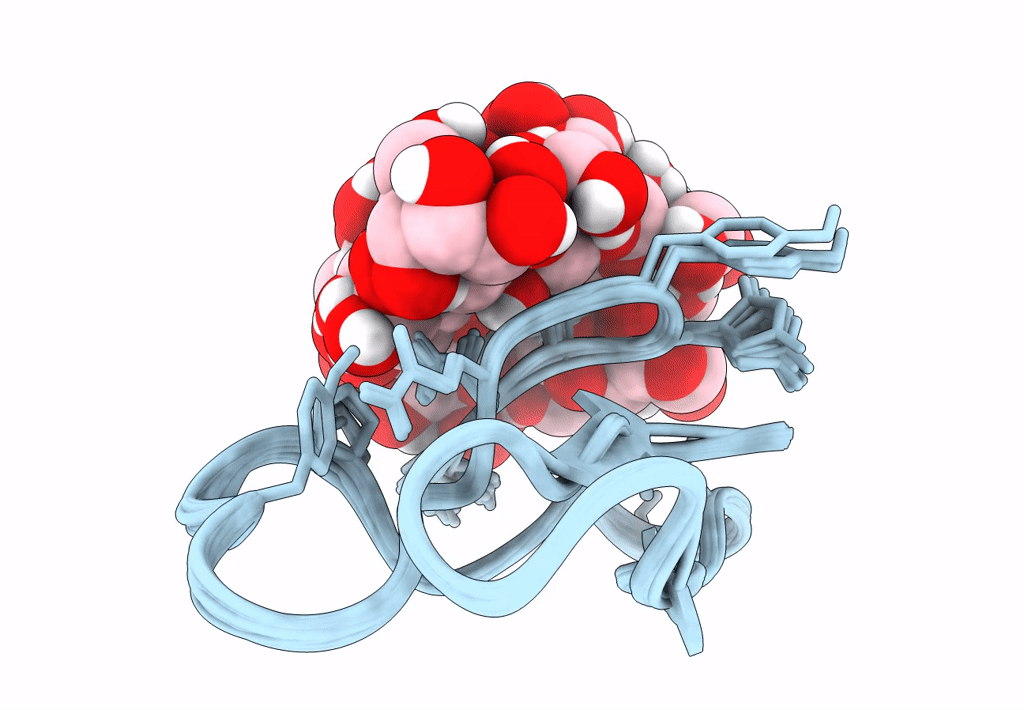
Solution structure of S-di-mannosylated S3C mutant of carbohydrate binding module (CBM) of the glycoside hydrolase Family 7 cellobiohydrolase from Trichoderma reesei
Biological Source:
Source Organism(s):
Trichoderma reesei (Taxon ID: 51453)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy


