Deposition Date
2022-06-25
Release Date
2023-02-15
Last Version Date
2024-05-29
Entry Detail
PDB ID:
7Y9P
Keywords:
Title:
Xylitol dehydrogenase S96C/S99C/Y102C mutant(thermostabilized form) from Pichia stipitis
Biological Source:
Source Organism(s):
Scheffersomyces stipitis (Taxon ID: 4924)
Expression System(s):
Method Details:
Experimental Method:
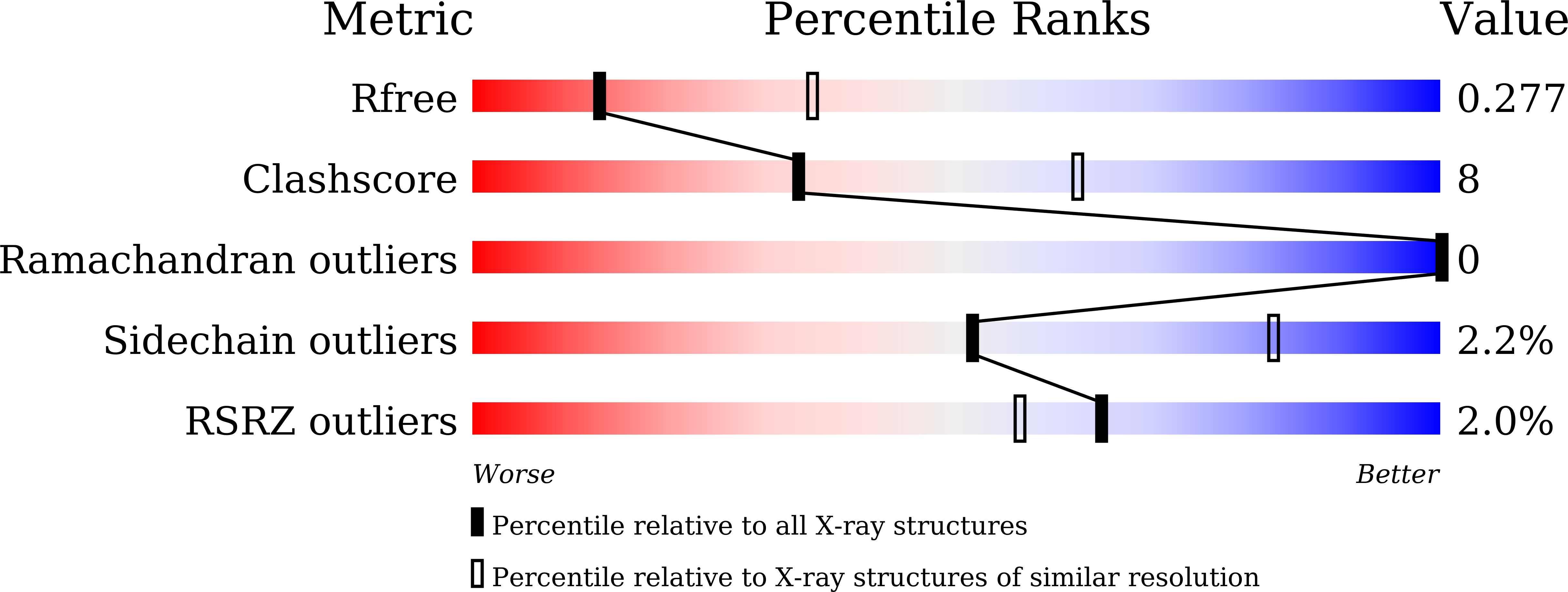
Resolution:
2.80 Å
R-Value Free:
0.27
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 6 2 2