Deposition Date
2022-04-28
Release Date
2023-01-11
Last Version Date
2024-11-13
Entry Detail
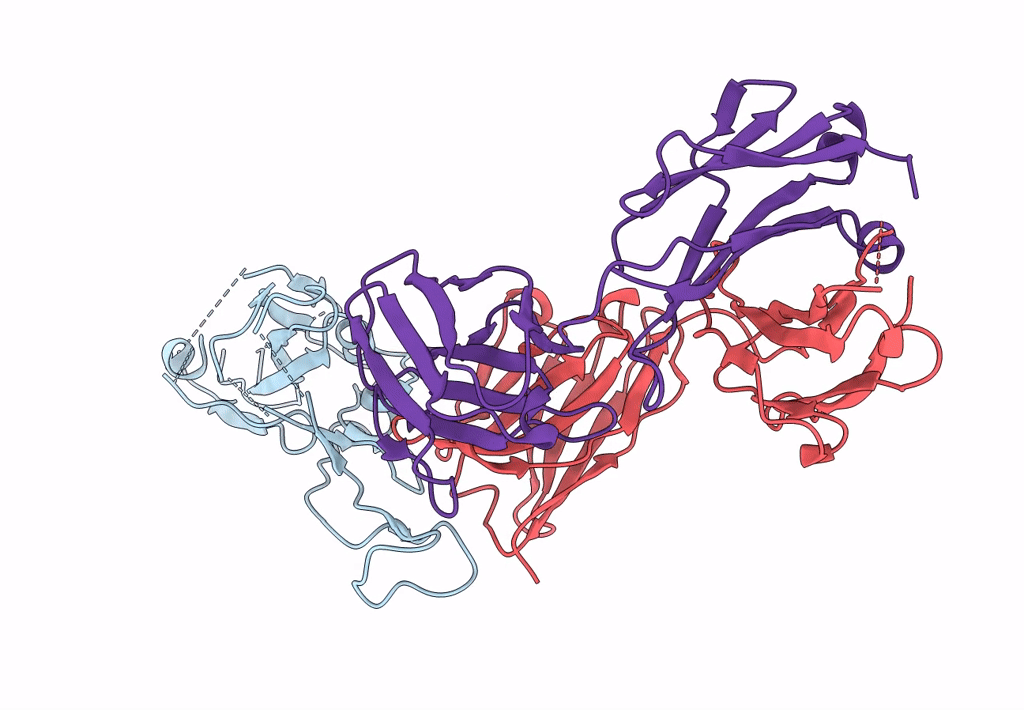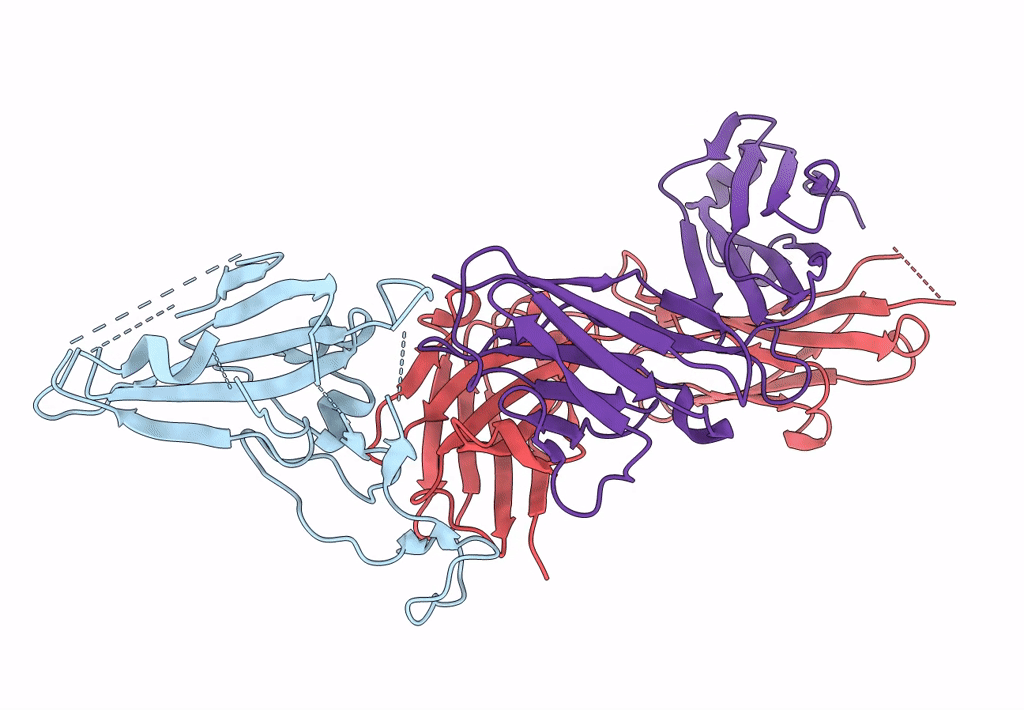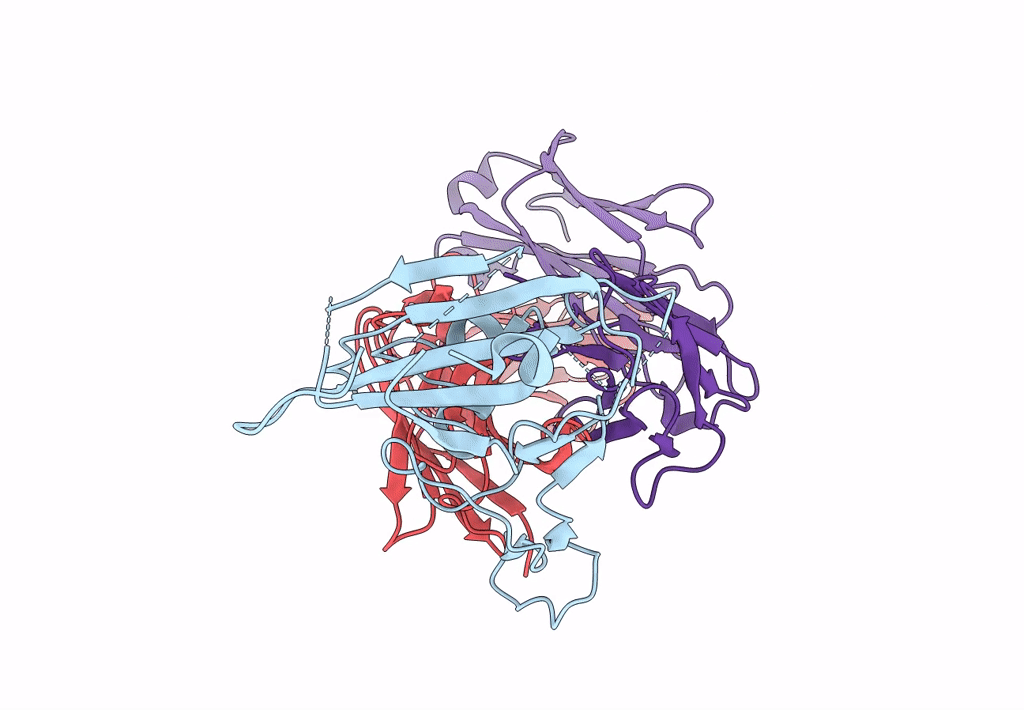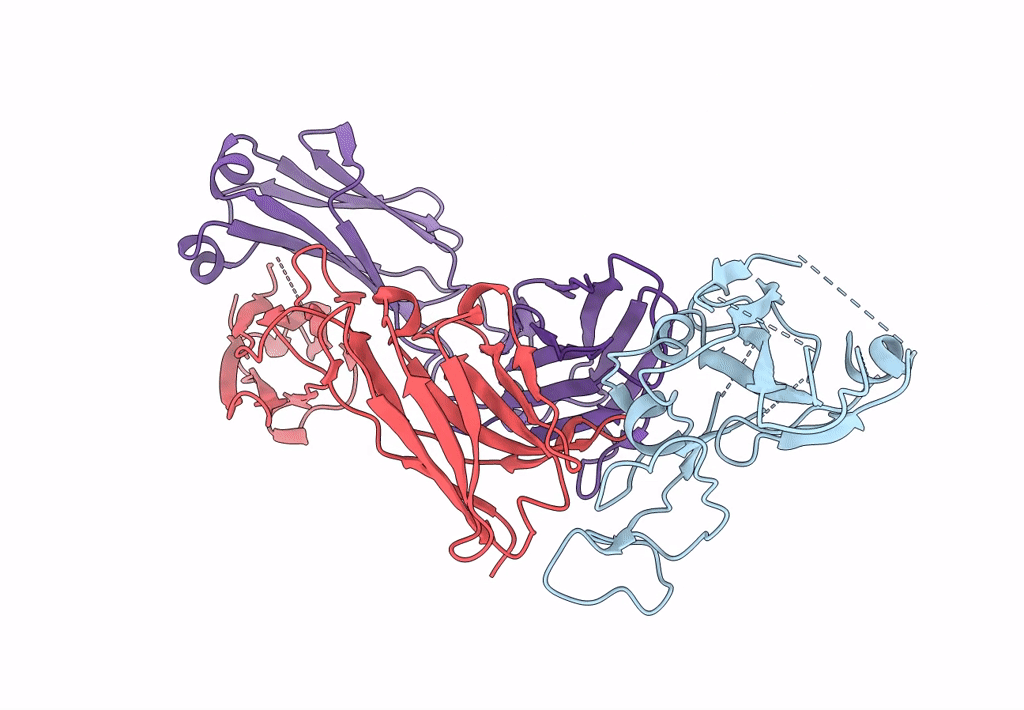
PDB ID:
7XNF
Keywords:
Title:
Structure of SARS-CoV-2 antibody P2C-1F11 with GX/P2V/2017 RBD
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Pangolin coronavirus (Taxon ID: 2708335)
Pangolin coronavirus (Taxon ID: 2708335)
Expression System(s):
Method Details:
Experimental Method:
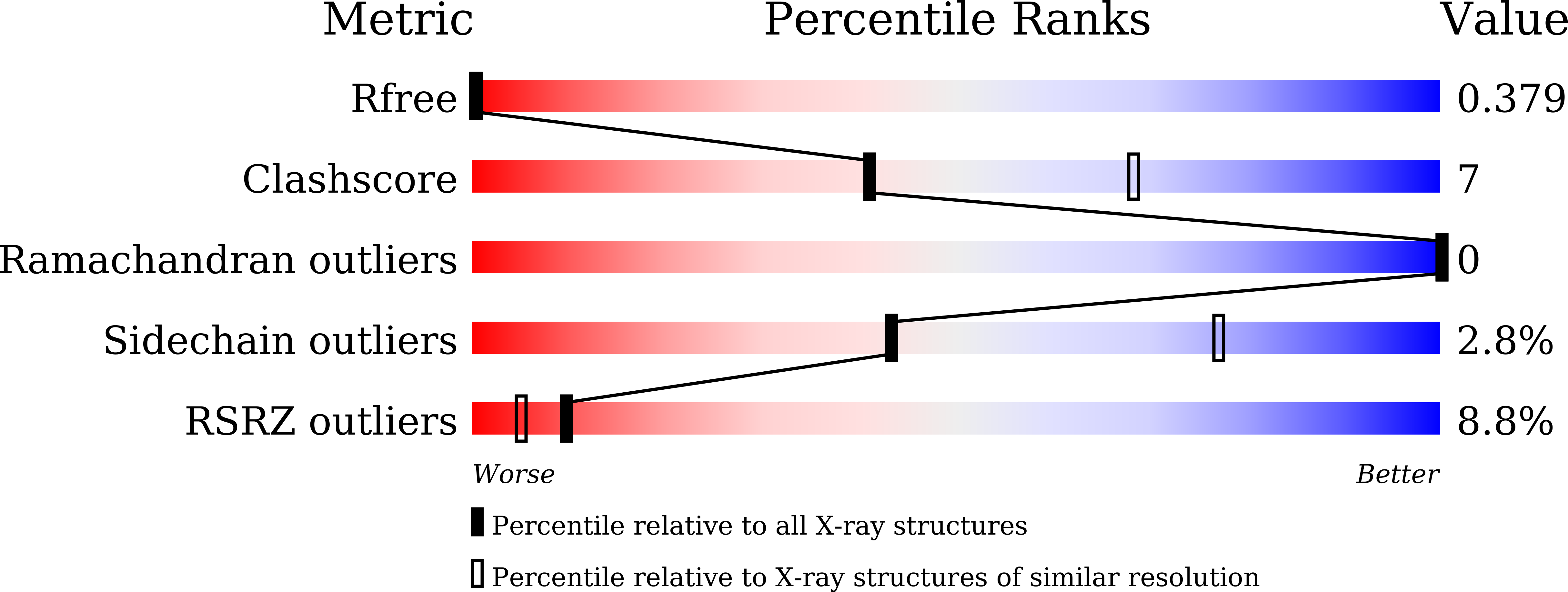
Resolution:
2.79 Å
R-Value Free:
0.37
R-Value Work:
0.31
R-Value Observed:
0.32
Space Group:
P 21 21 21