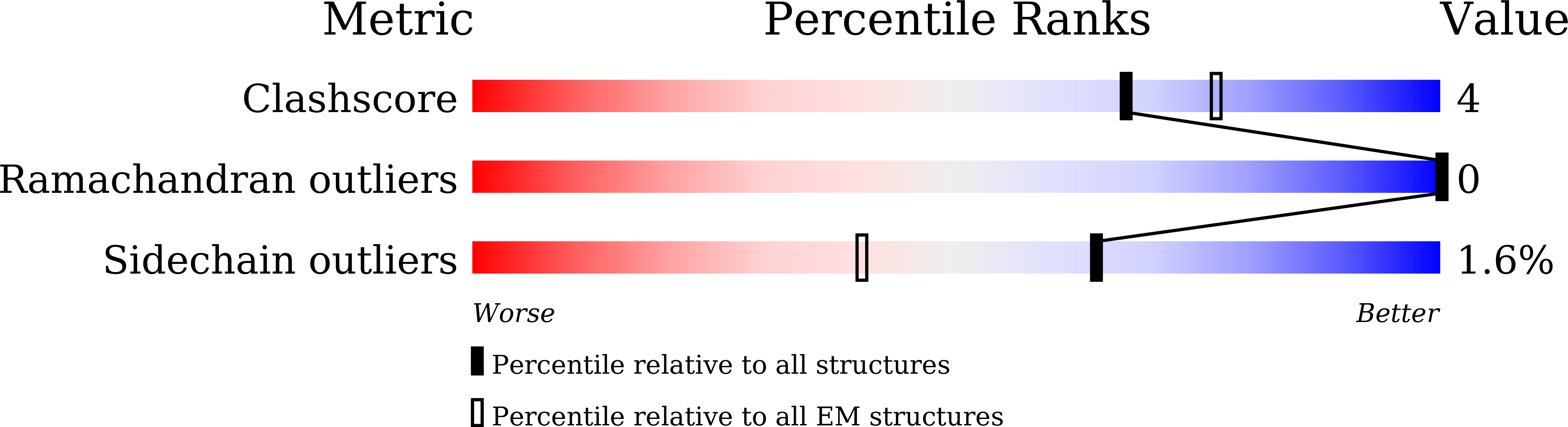
Deposition Date
2022-04-26
Release Date
2022-06-29
Last Version Date
2024-07-03
Entry Detail
PDB ID:
7XMU
Keywords:
Title:
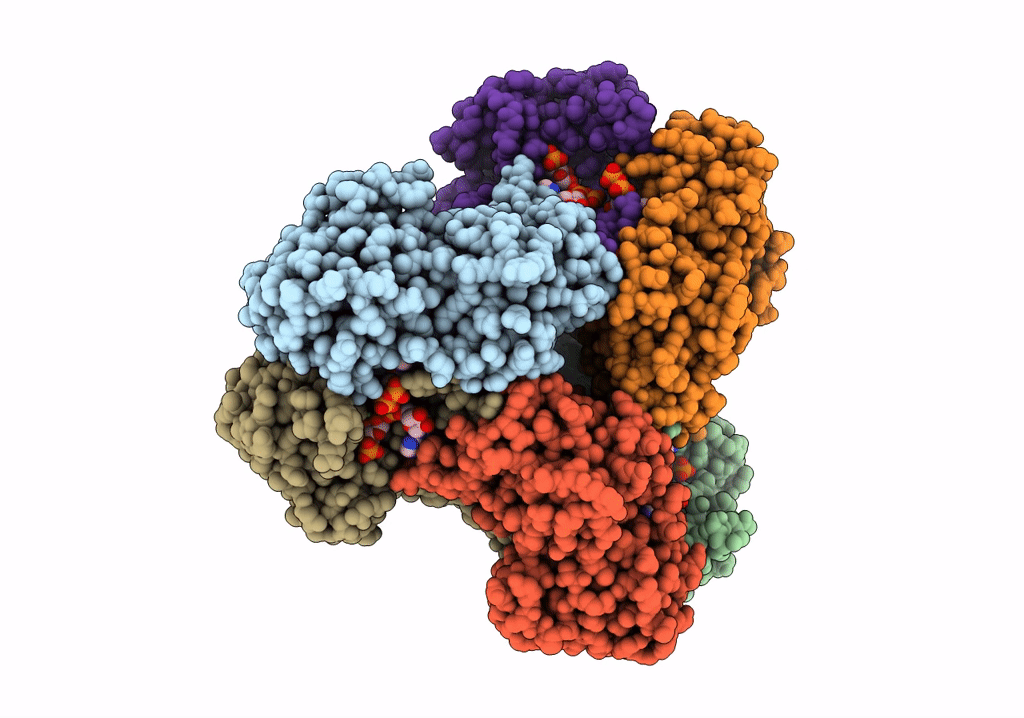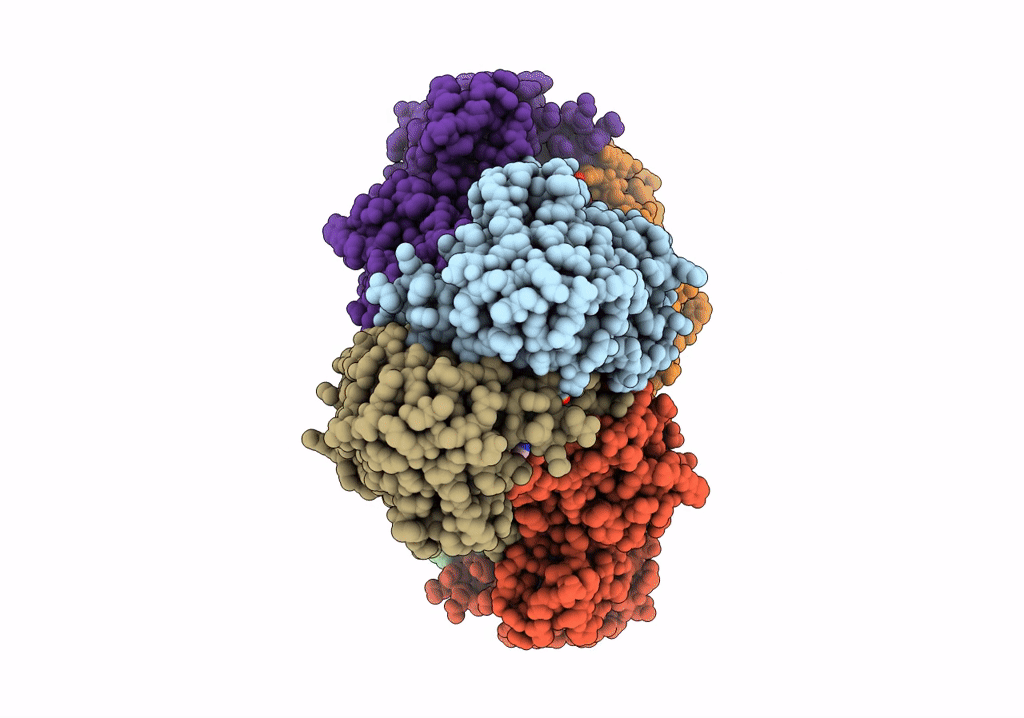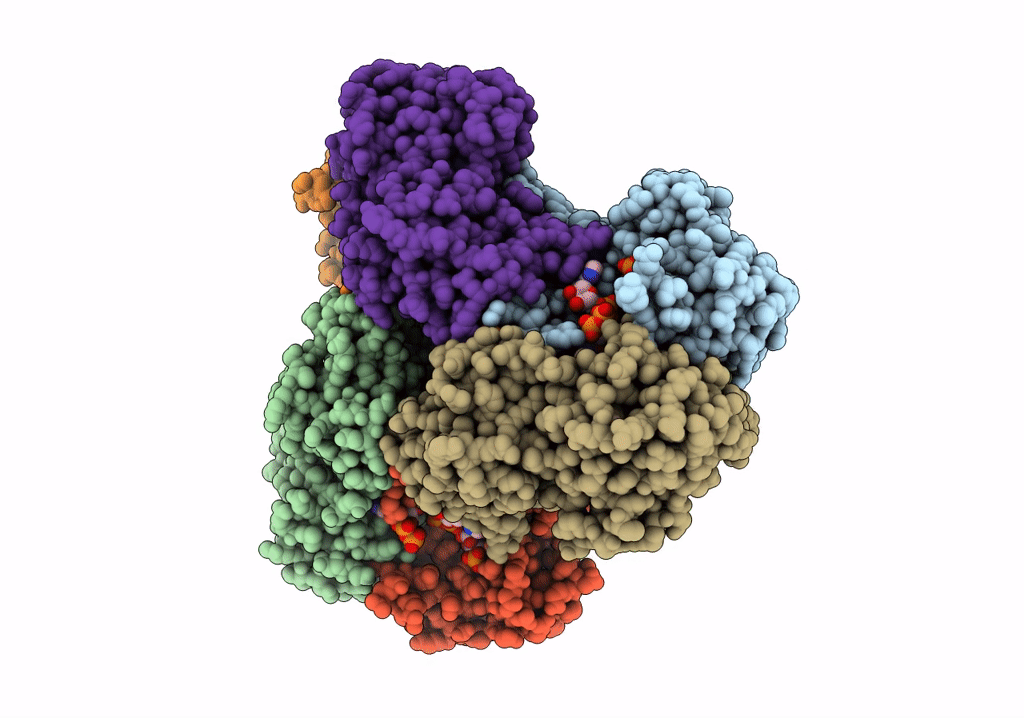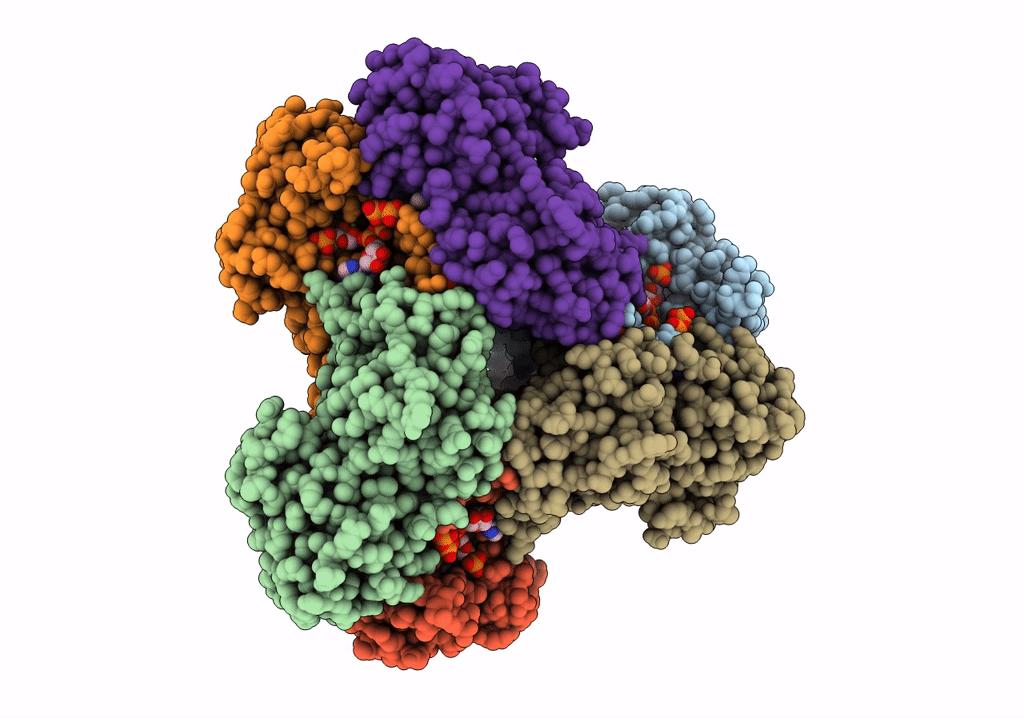
E.coli phosphoribosylpyrophosphate (PRPP) synthetase type A filament bound with ADP, Pi and R5P
Biological Source:
Source Organism(s):
Escherichia coli str. K-12 substr. MG1655 (Taxon ID: 511145)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.30 Å
Aggregation State:
FILAMENT
Reconstruction Method:
SINGLE PARTICLE