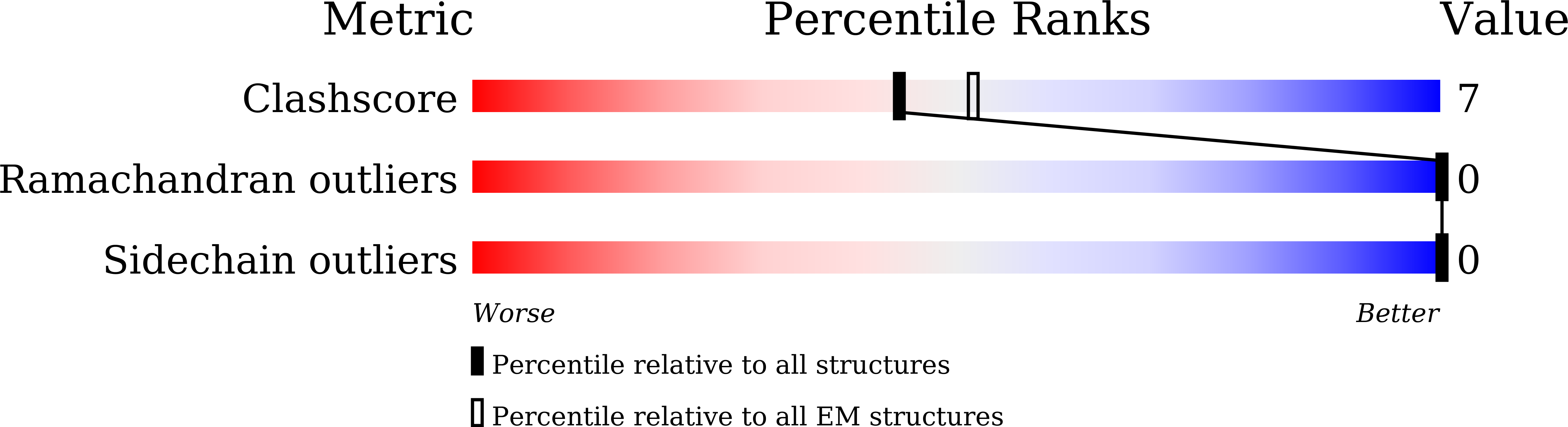
Deposition Date
2022-04-18
Release Date
2022-05-04
Last Version Date
2025-07-02
Entry Detail
PDB ID:
7XJH
Keywords:
Title:
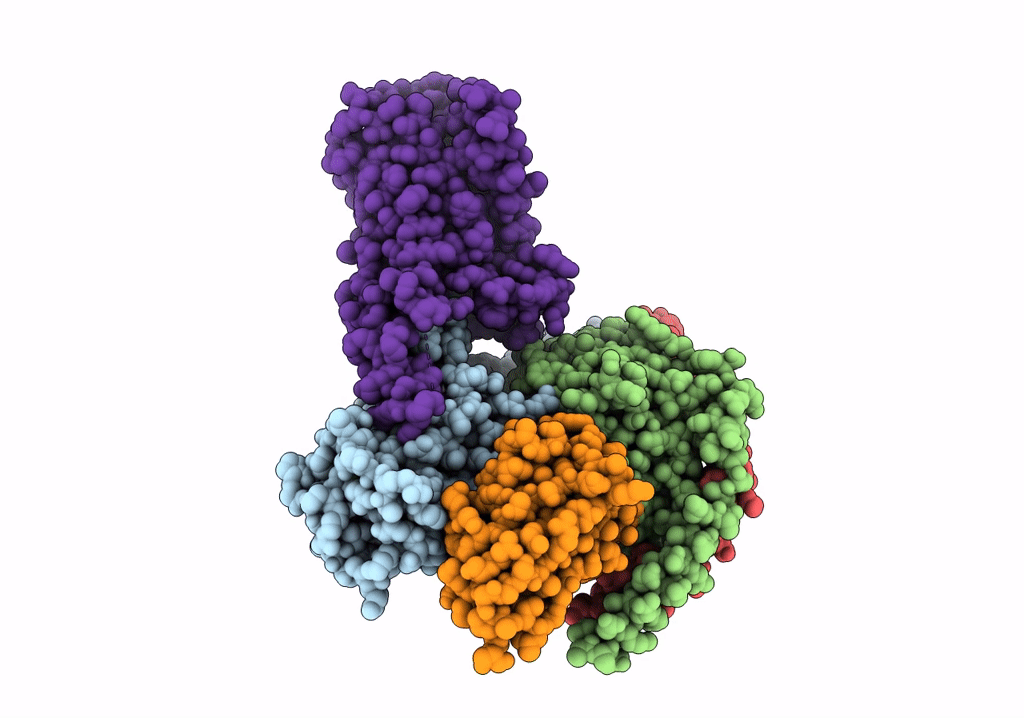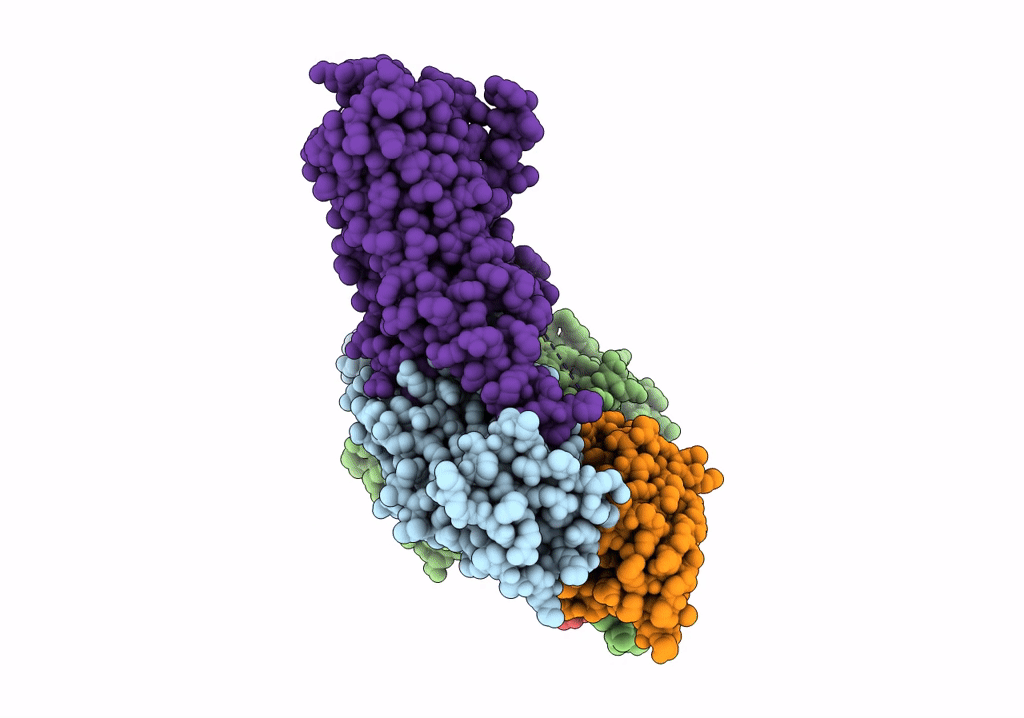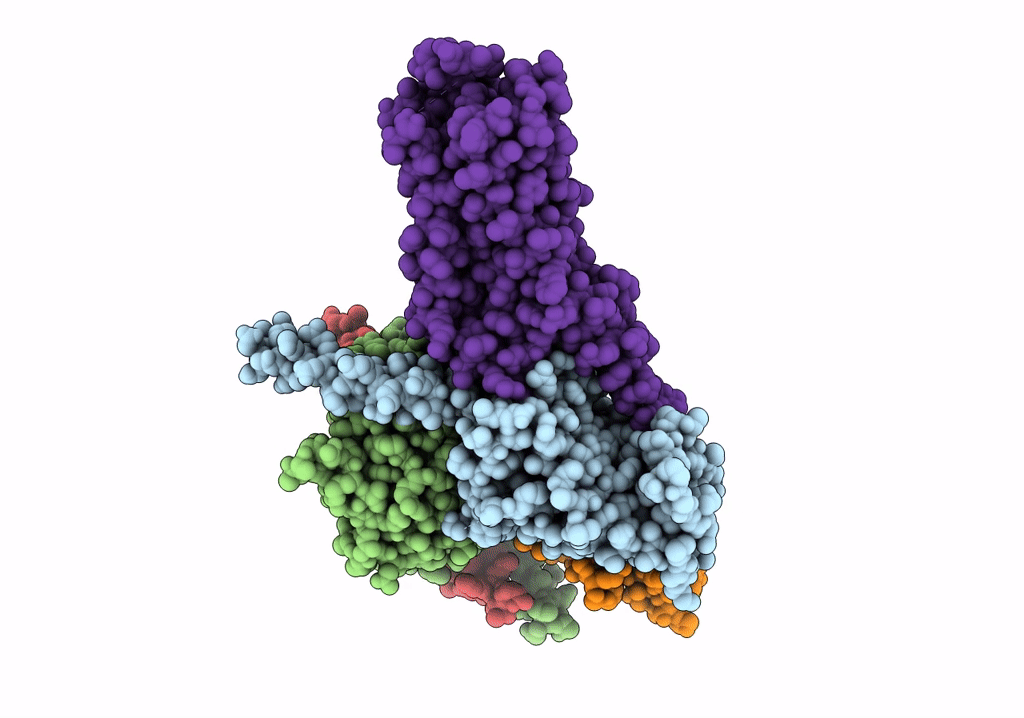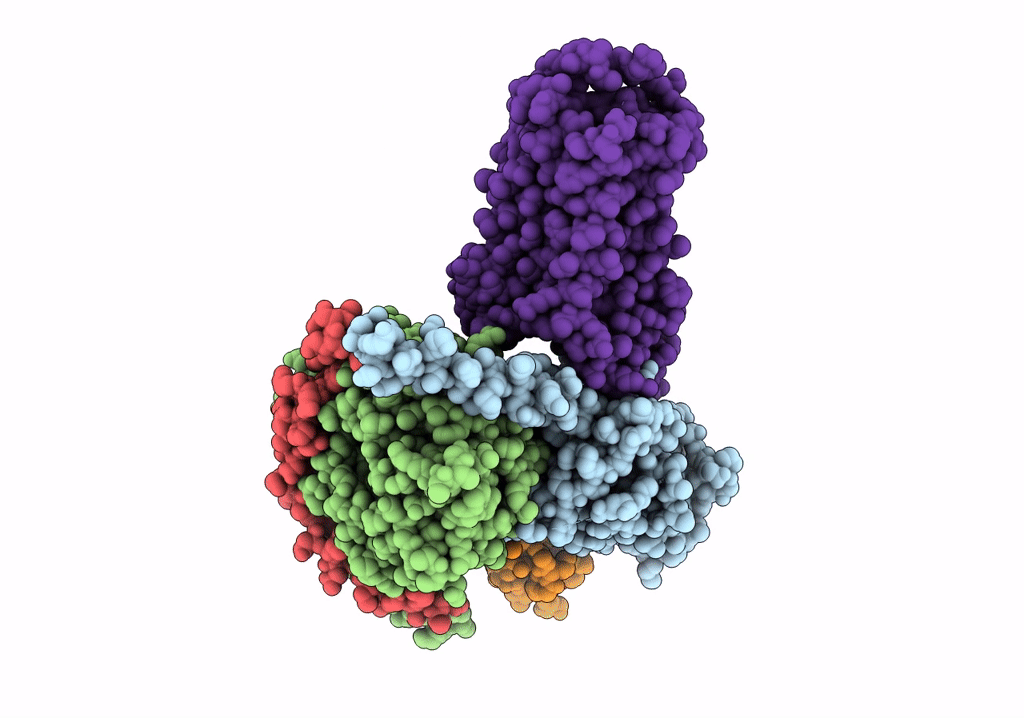
Isoproterenol-activated dog beta3 adrenergic receptor
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Rattus norvegicus (Taxon ID: 10116)
Bos taurus (Taxon ID: 9913)
unidentified (Taxon ID: 32644)
Canis lupus familiaris (Taxon ID: 9615)
Rattus norvegicus (Taxon ID: 10116)
Bos taurus (Taxon ID: 9913)
unidentified (Taxon ID: 32644)
Canis lupus familiaris (Taxon ID: 9615)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.30 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE