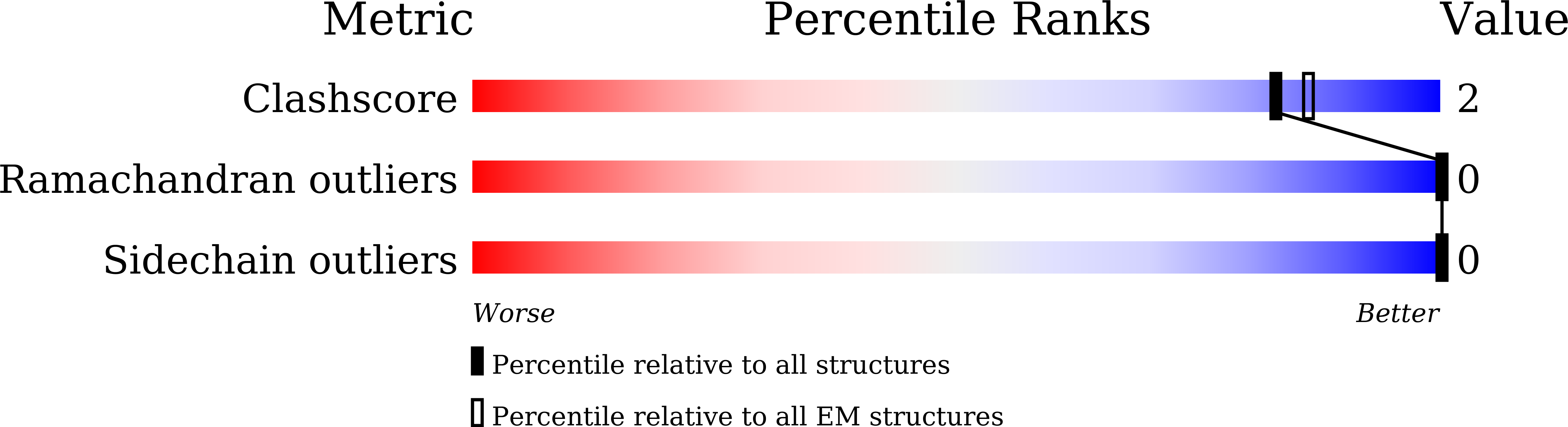
Deposition Date
2022-02-26
Release Date
2022-09-28
Last Version Date
2025-06-25
Entry Detail
PDB ID:
7X2T
Keywords:
Title:
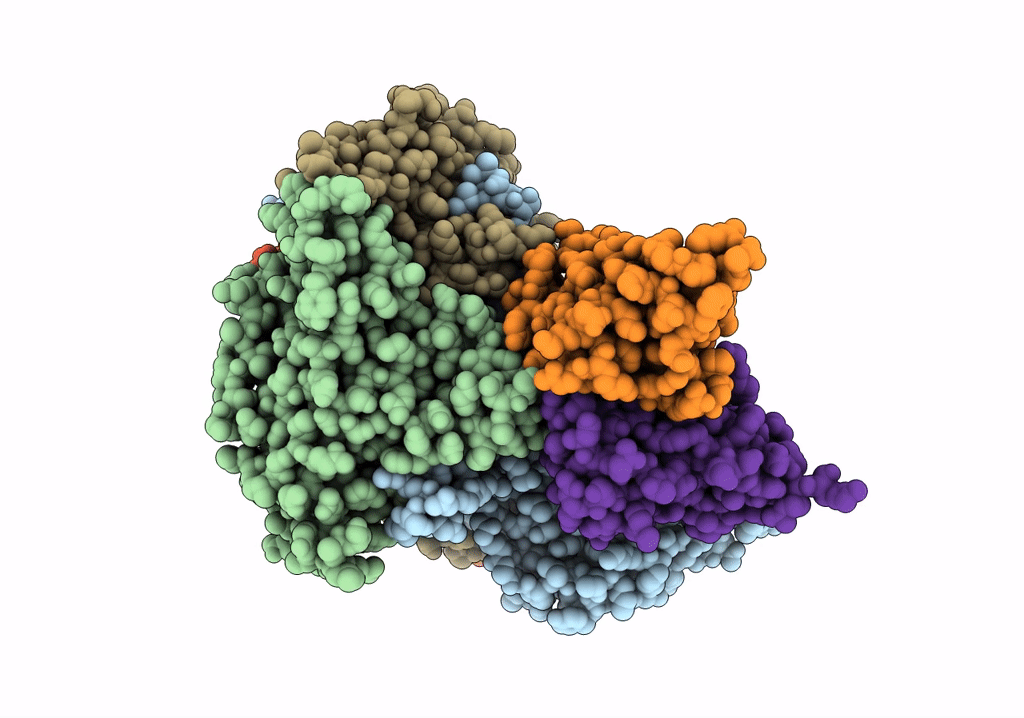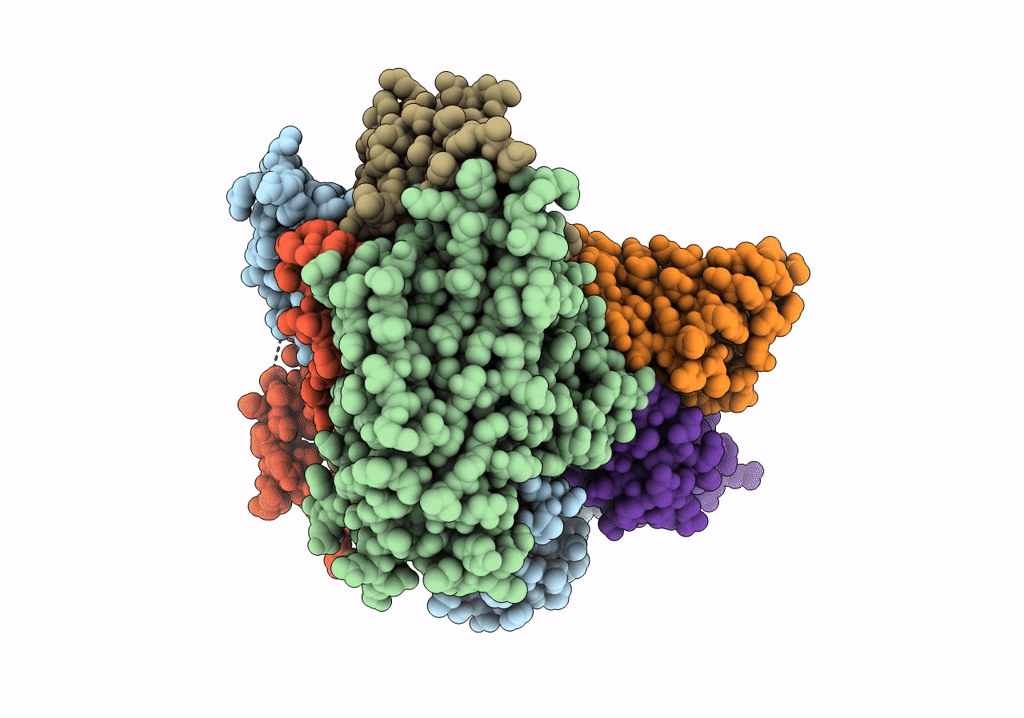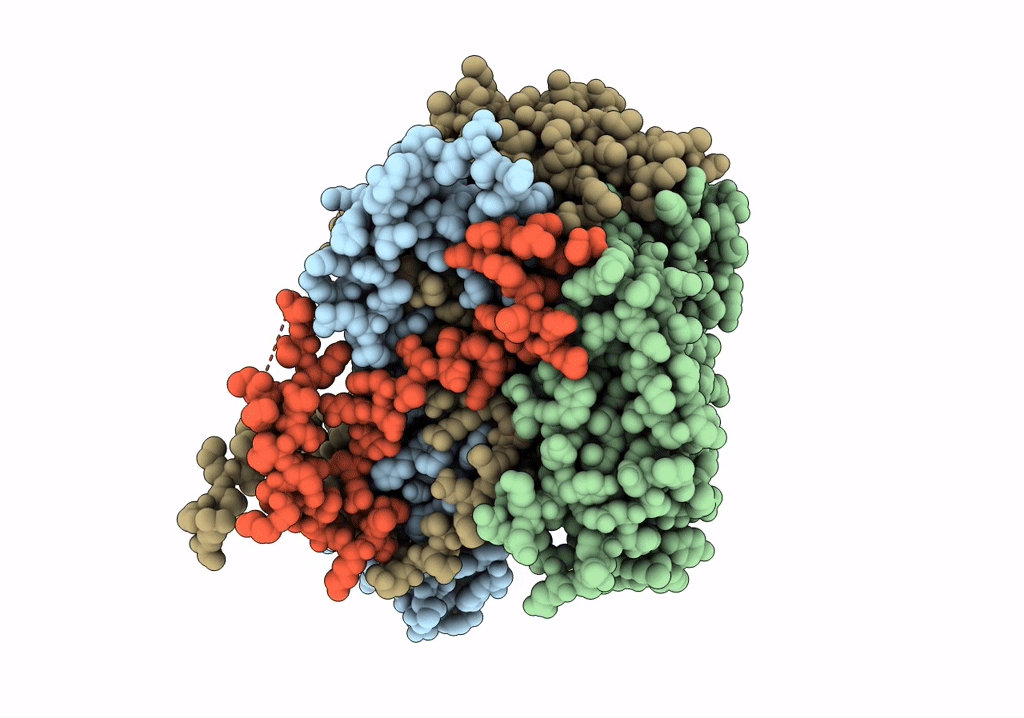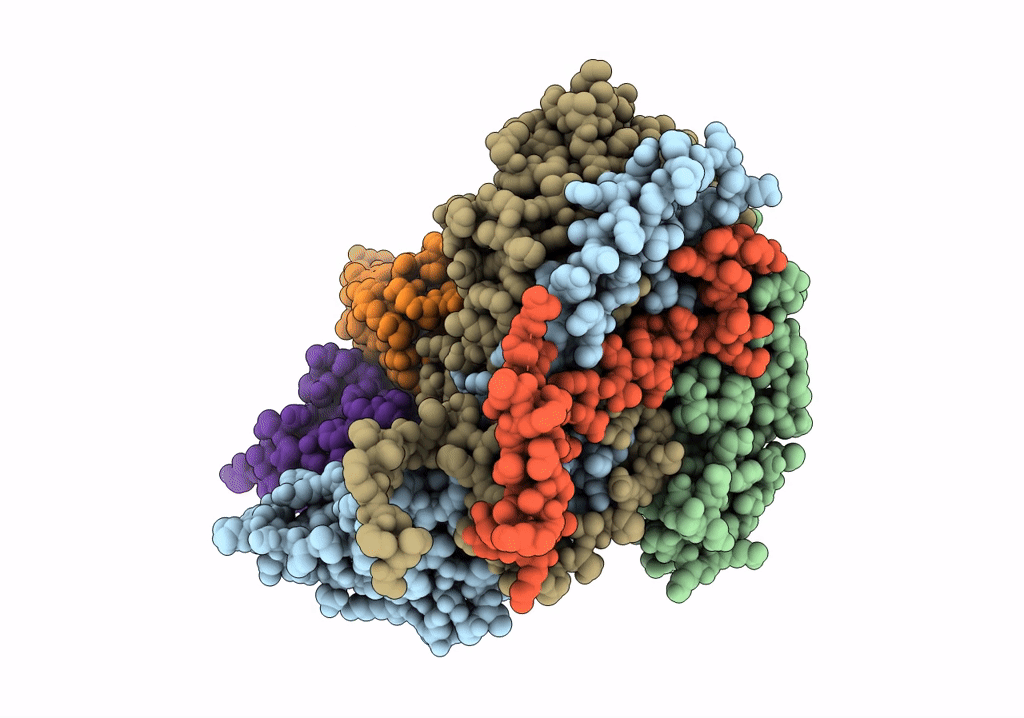
Cryo-EM structure of Coxsackievirus B1 mature virion in complex with nAb 8A10 (CVB1-M:8A10)
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Coxsackievirus B1 (Taxon ID: 12071)
Coxsackievirus B1 (Taxon ID: 12071)
Method Details:
Experimental Method:
Resolution:
3.69 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE