Deposition Date
2022-02-16
Release Date
2022-04-27
Last Version Date
2022-04-27
Entry Detail
PDB ID:
7WYU
Keywords:
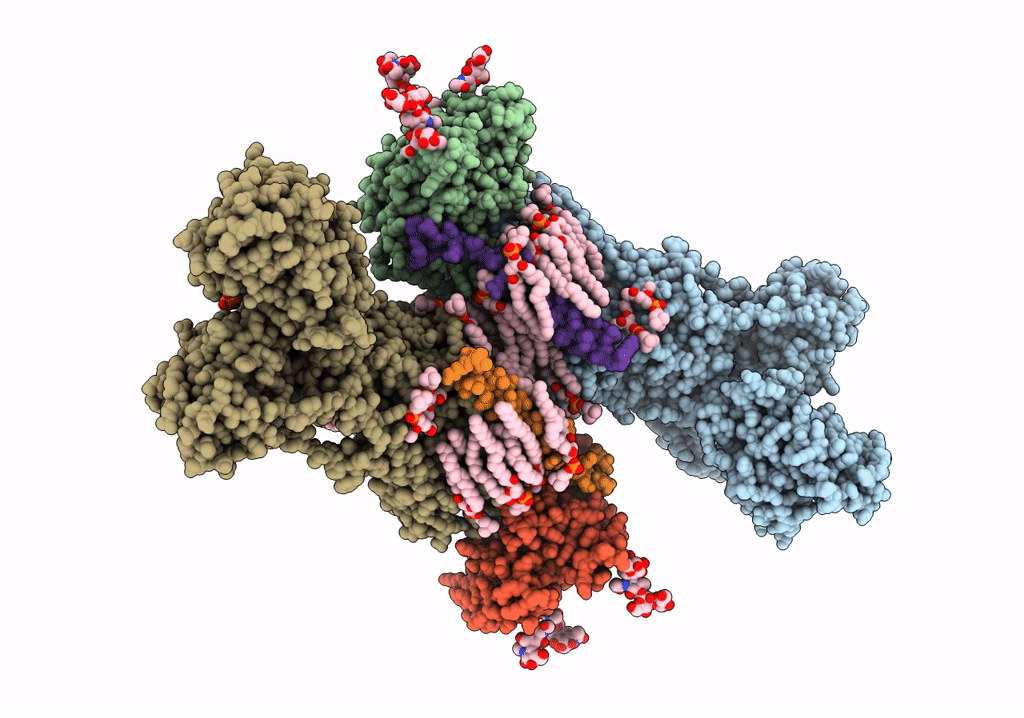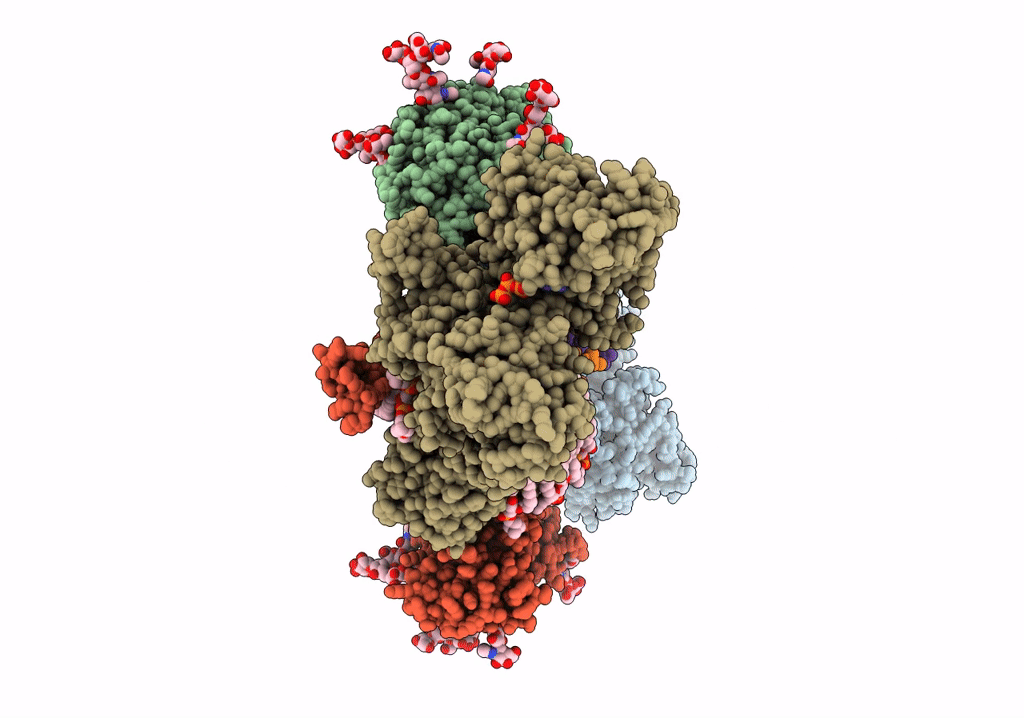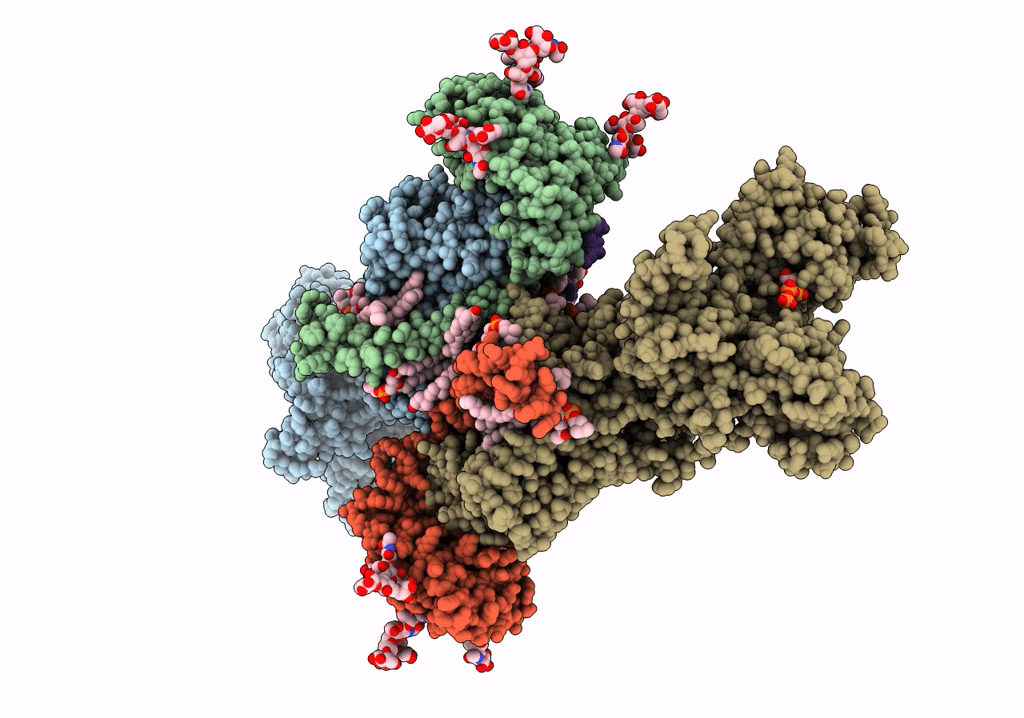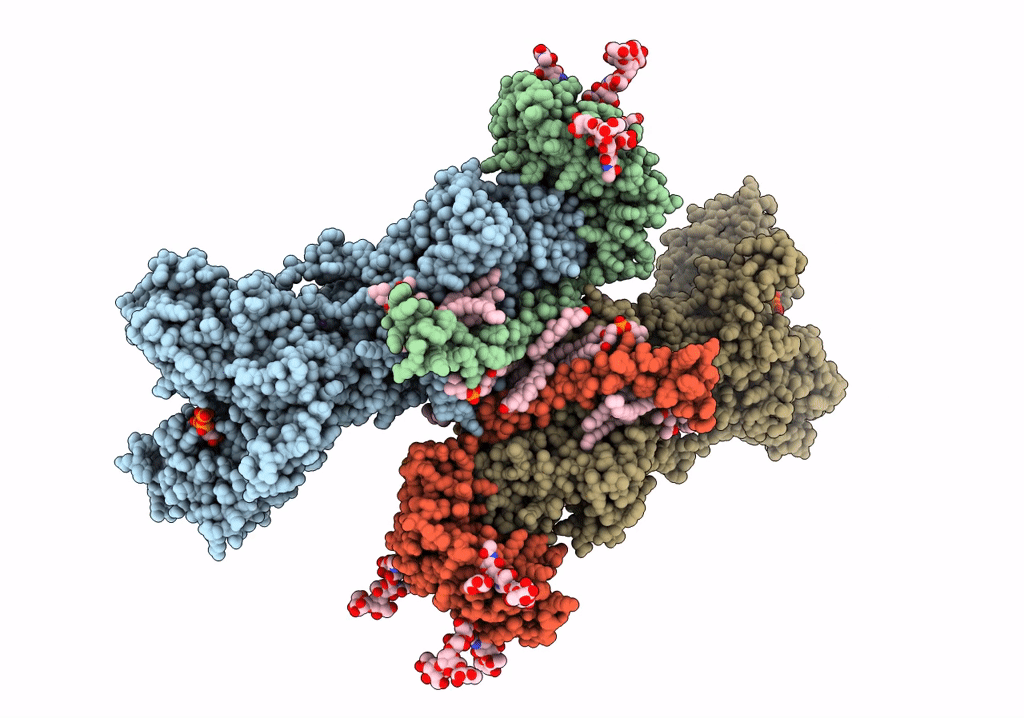
Title:
Cryo-EM structure of Na+,K+-ATPase in the E2P state formed by ATP
Biological Source:
Source Organism(s):
Squalus acanthias (Taxon ID: 7797)
Method Details:
Experimental Method:
Resolution:
3.40 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


