Abstact
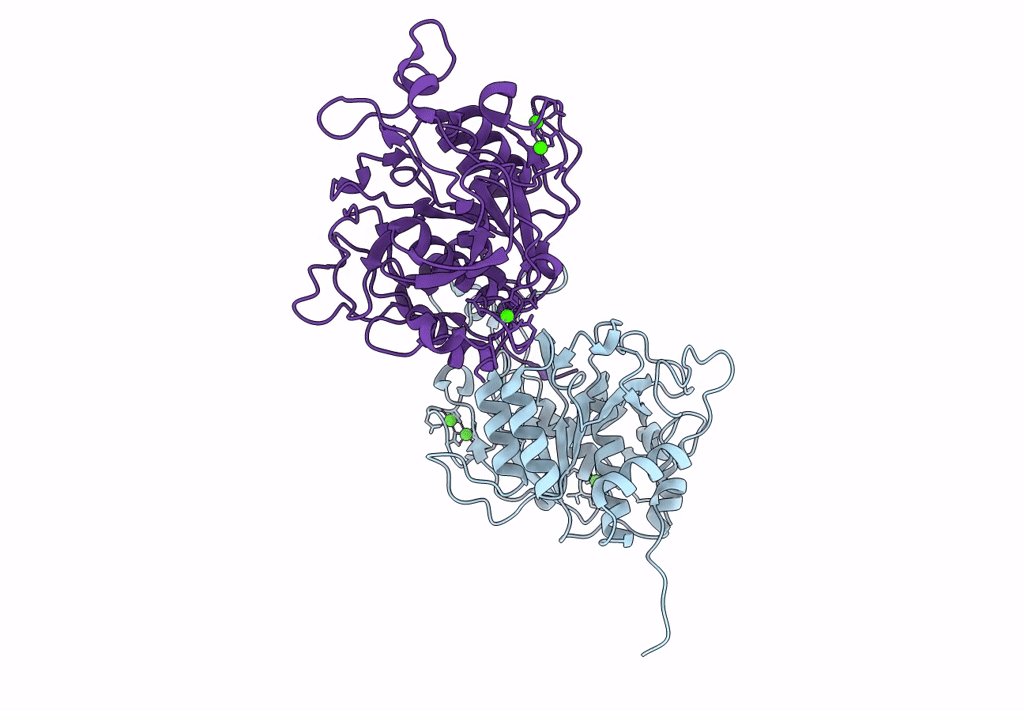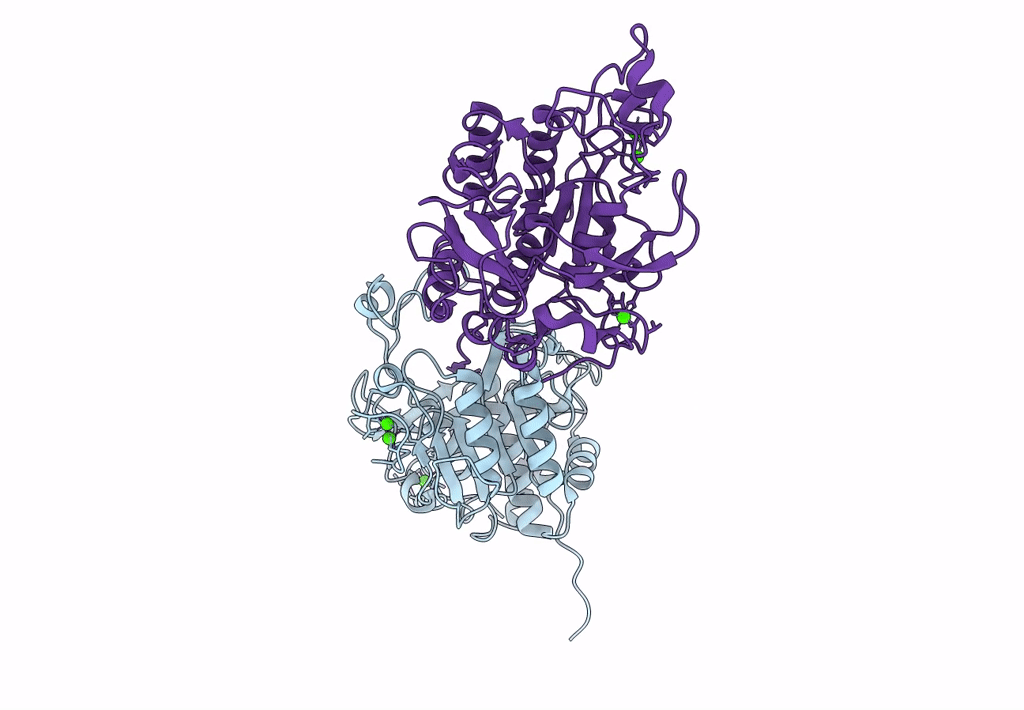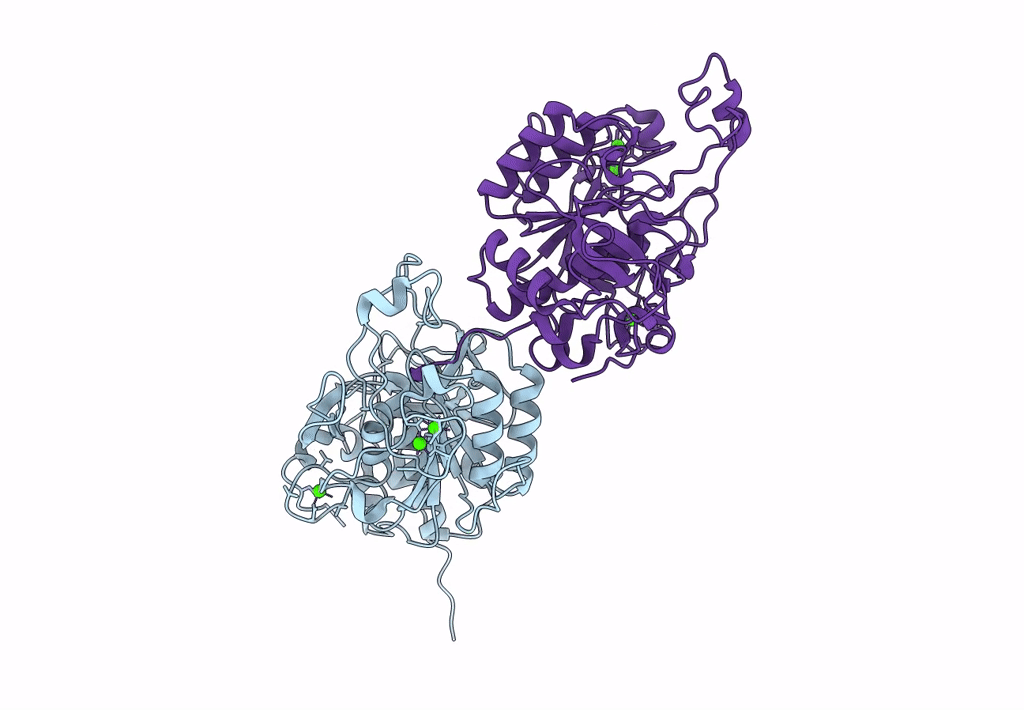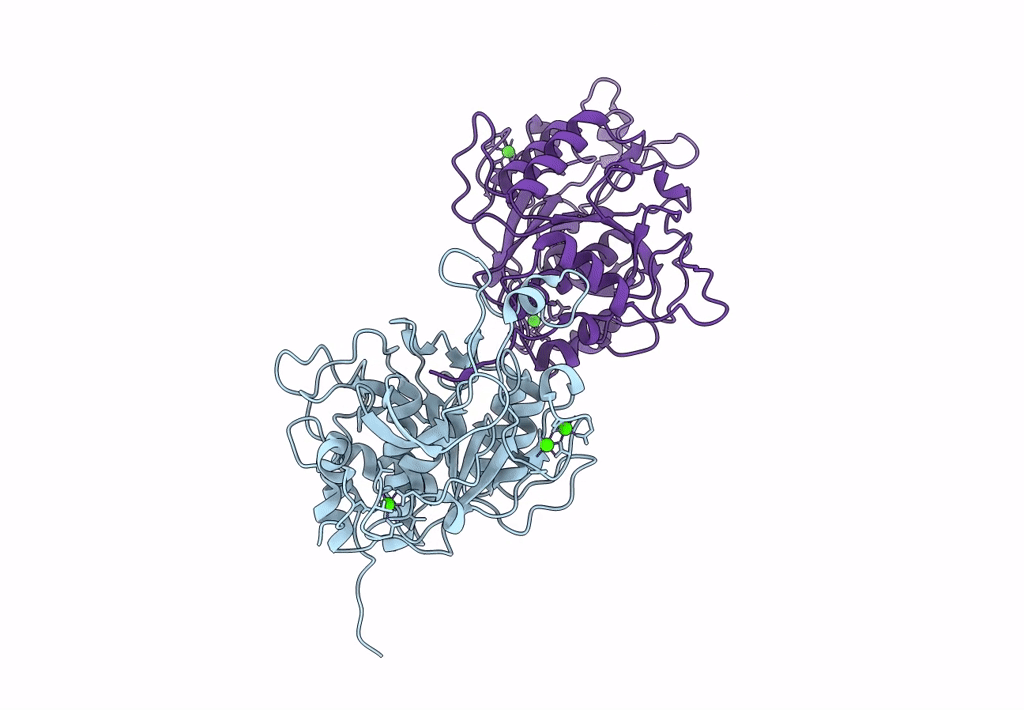
Celiac disease (CD) is a human autoimmune disease triggered by toxic gluten peptides. Recently, oral enzyme therapy has been proposed to ameliorate the health condition of CD patients based on the concept of removing pepsin-insensitive gluten-derived pro-immunogenic peptides. A Burkholderia peptidase, Bga1903, with promising gluten-degrading activity was characterized previously. Here, we report the crystal structure of Bga1903, in which the core has a α/β/α fold featured with a twisted six-stranded parallel β-sheet sandwiched between two layers of α-helices. The mutations at the substrate-binding pocket that might enhance the peptidase's affinity toward tetrapeptide PQPQ were predicted by FoldX. Accordingly, four single-substitution mutants, G351A, E380L, S386F, and S387L, were created. The specificity constant (kcat/KM) of wild type toward chromogenic peptidyl substrates Z-HPK-pNA, Z-HPQ-pNA, Z-HPL-pNA, and Z-QPQ-pNA are 30.2, 7.9, 3.3, and 0.79 s-1·mM-1, respectively, indicating that the QPQ motif, which frequently occurs in pro-immunogenic peptides, is not favorable. Among the mutants, E380L loses the hydrolytic activity toward Z-HPK-pNA, suggesting a critical role of E380 in preferring a lysine residue at the P1 position. S387L shows a 17-fold increase in the specificity constant toward Z-QPQ-pNA and hydrolyzes the pro-immunogenic peptides more efficiently than the wild-type peptidase.