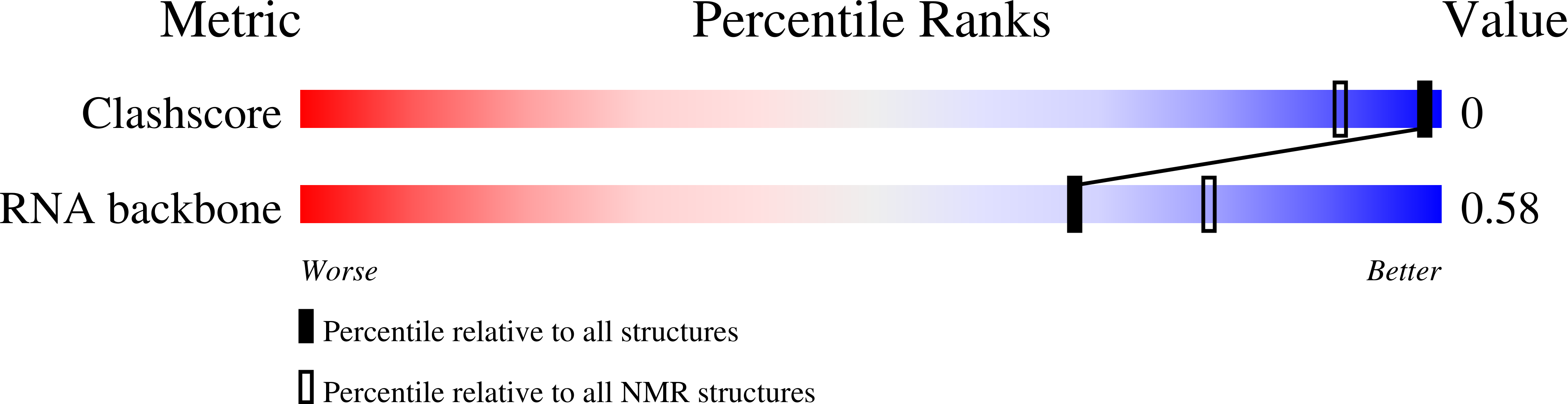
Deposition Date
2022-05-09
Release Date
2022-06-29
Last Version Date
2024-05-15
Entry Detail
PDB ID:
7V06
Keywords:
Title:
Encoded Conformational Dynamics of the HIV Splice Site A3 Regulatory Locus: Implications for differential binding of hnRNP Splicing Auxiliary Factors
Biological Source:
Source Organism(s):
HIV whole-genome vector AA1305#18 (Taxon ID: 672471)
Method Details:
Experimental Method:
Conformers Calculated:
1024
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy