Deposition Date
2022-05-05
Release Date
2022-10-12
Last Version Date
2023-10-18
Entry Detail
PDB ID:
7UX7
Keywords:
Title:
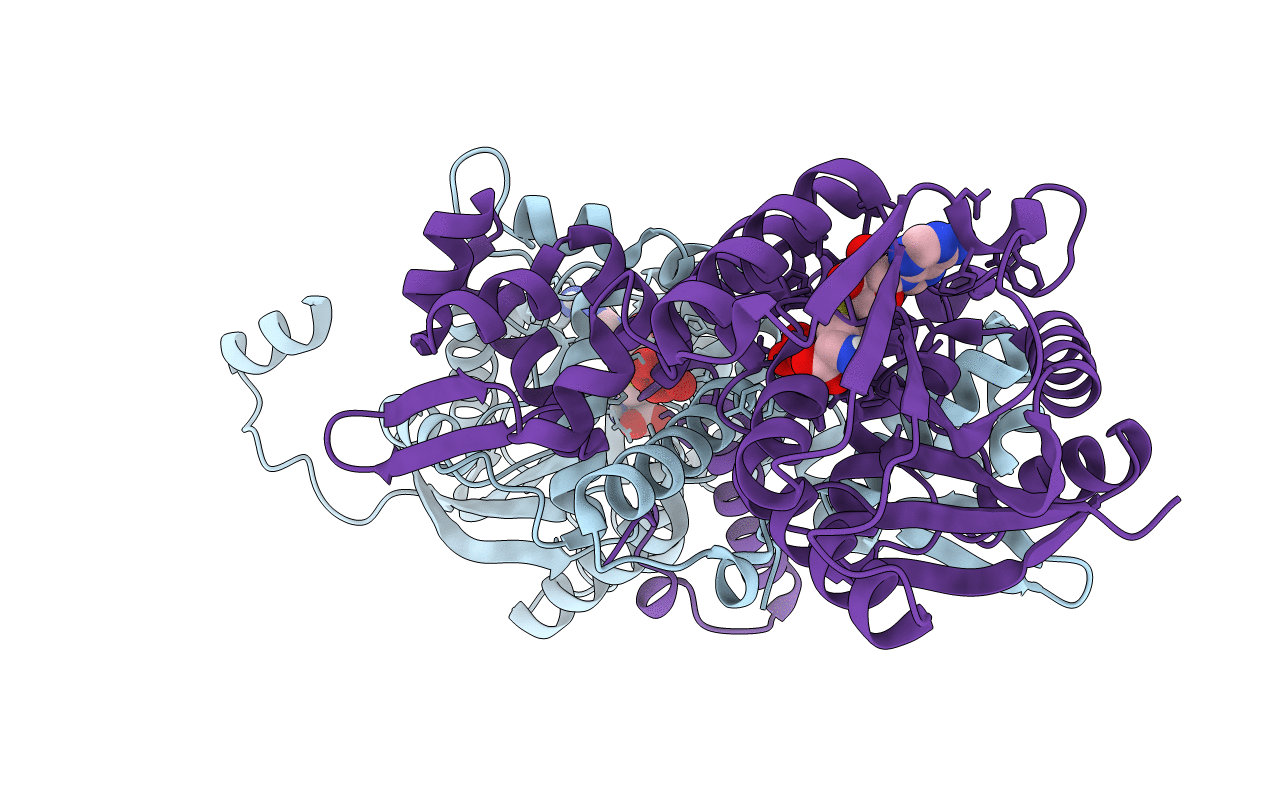
Crystal structure of MfnG, an L- and D-tyrosine O-methyltransferase from the marformycin biosynthesis pathway of Streptomyces drozdowiczii, with SAH bound at 1.2 A resolution (P212121 - form II)
Biological Source:
Source Organism(s):
Streptomyces drozdowiczii (Taxon ID: 202862)
Expression System(s):
Method Details:
Experimental Method:
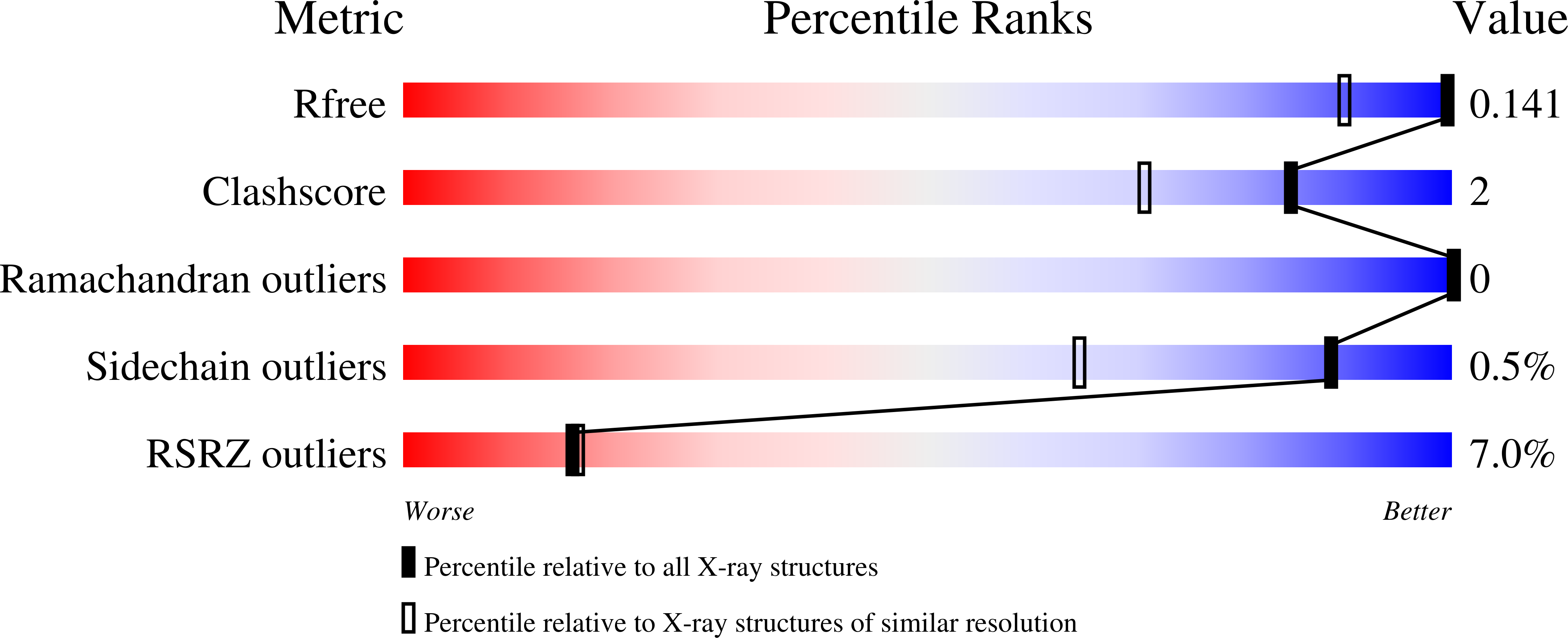
Resolution:
1.14 Å
R-Value Free:
0.14
R-Value Work:
0.11
R-Value Observed:
0.12
Space Group:
P 21 21 21