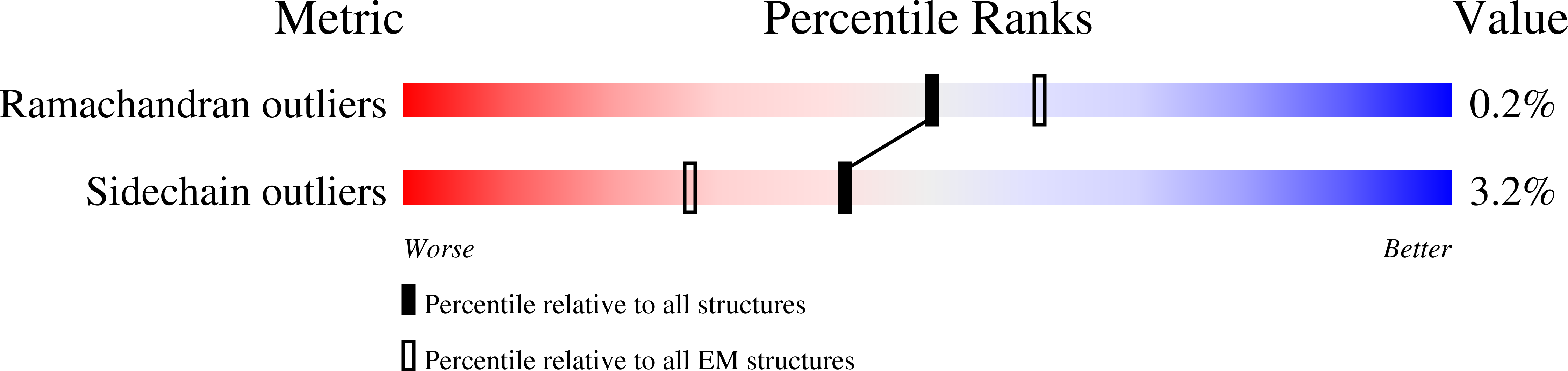
Deposition Date
2022-04-27
Release Date
2022-09-21
Last Version Date
2022-09-21
Entry Detail
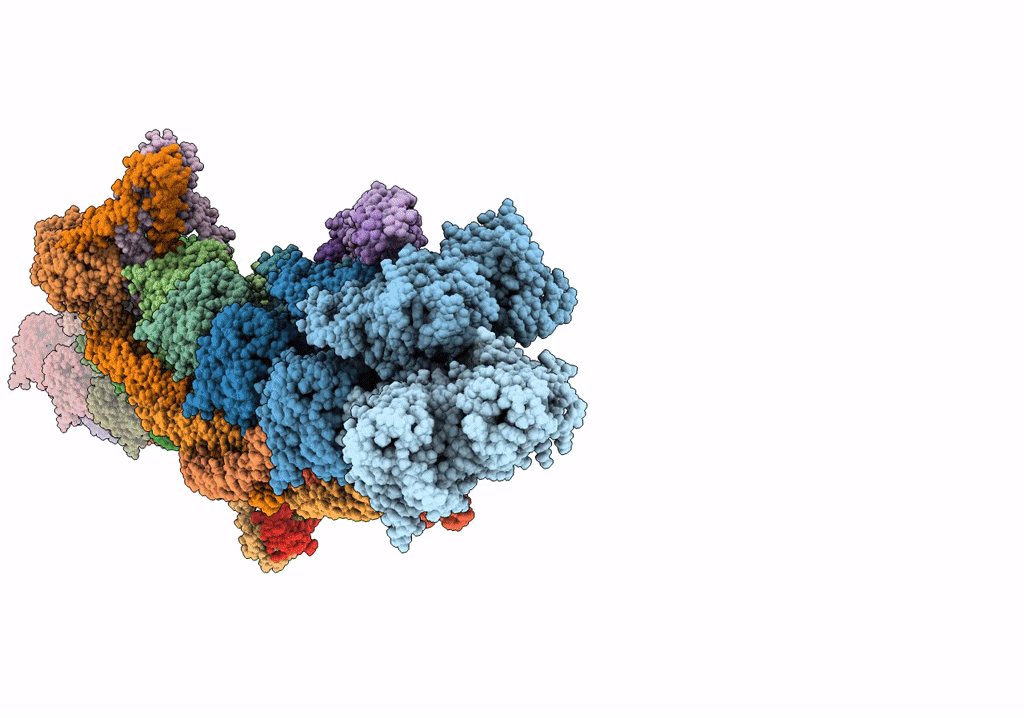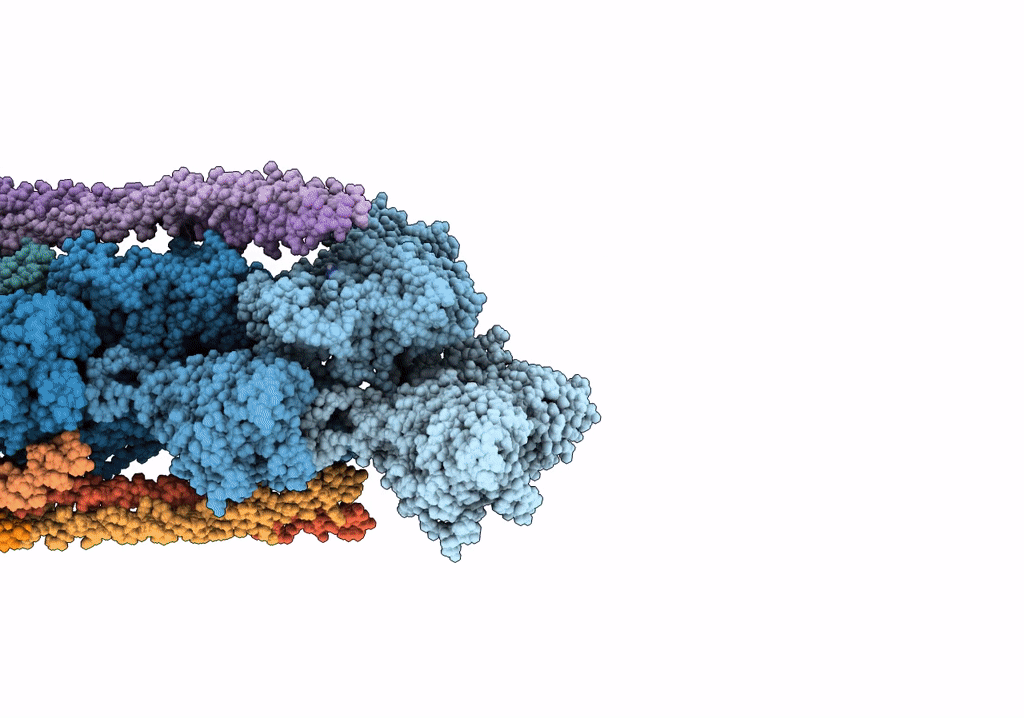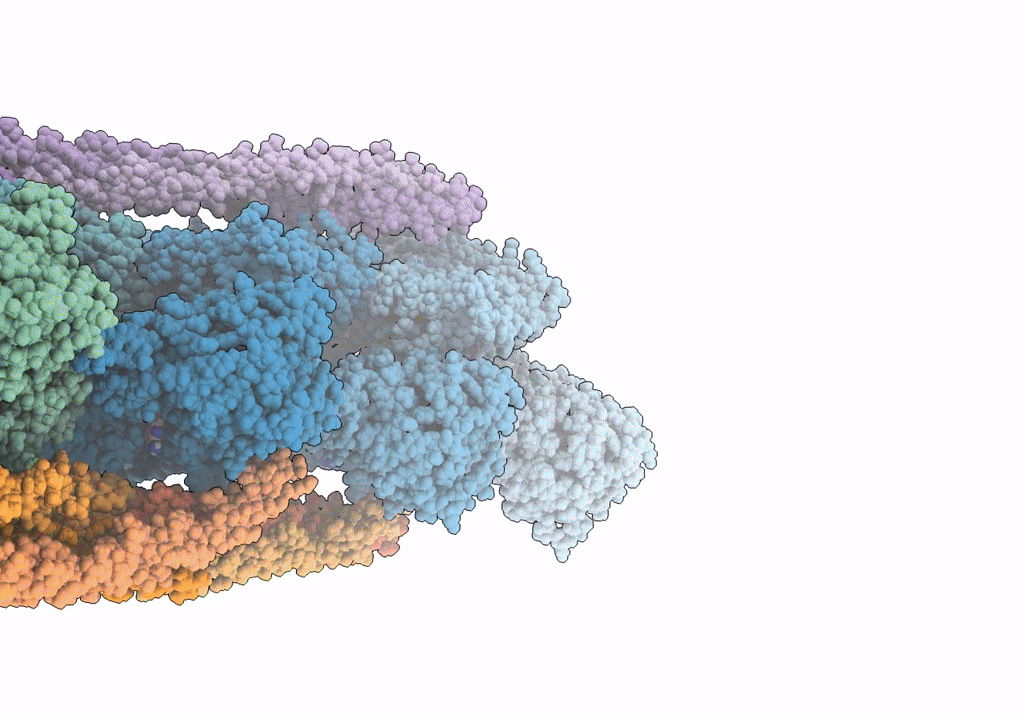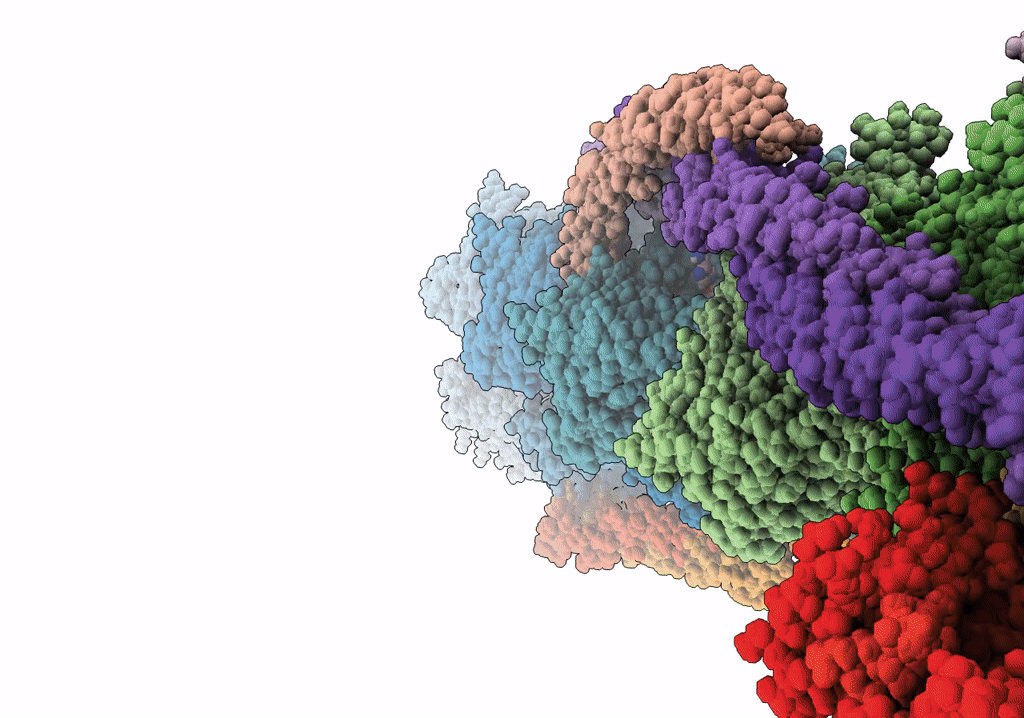
PDB ID:
7UTL
Keywords:
Title:
ALTERNATIVE MODELING OF TROPOMYOSIN IN HUMAN CARDIAC THIN FILAMENT IN THE CALCIUM FREE STATE
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Oryctolagus cuniculus (Taxon ID: 9986)
Oryctolagus cuniculus (Taxon ID: 9986)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
6.60 Å
Aggregation State:
FILAMENT
Reconstruction Method:
SINGLE PARTICLE