Deposition Date
2022-04-22
Release Date
2023-05-03
Last Version Date
2024-05-01
Entry Detail
PDB ID:
7URJ
Keywords:
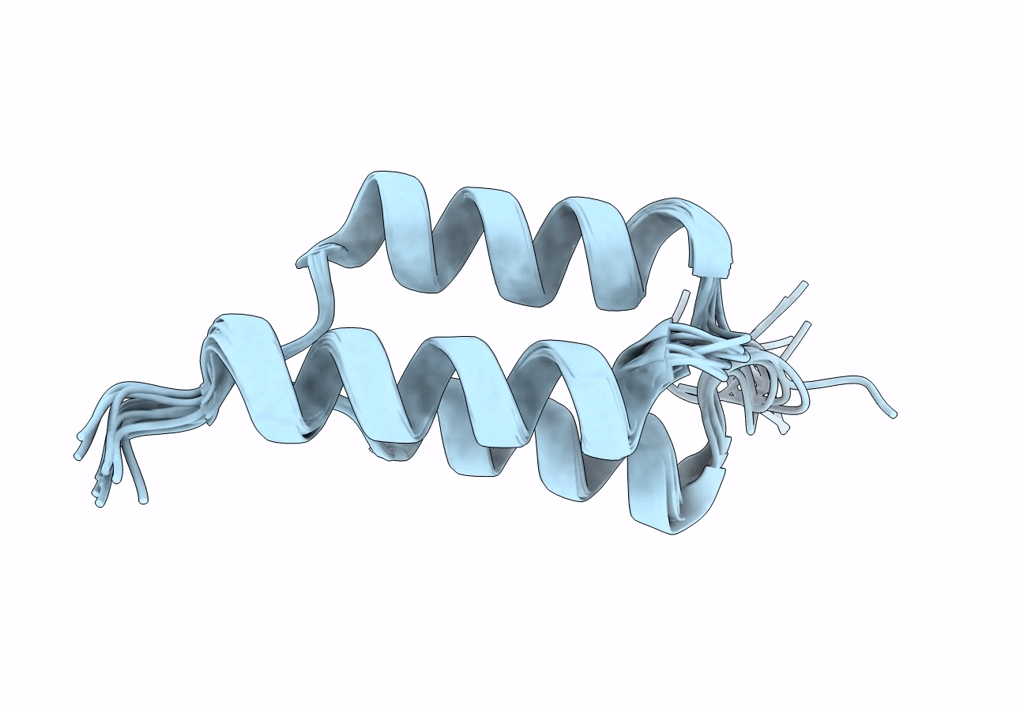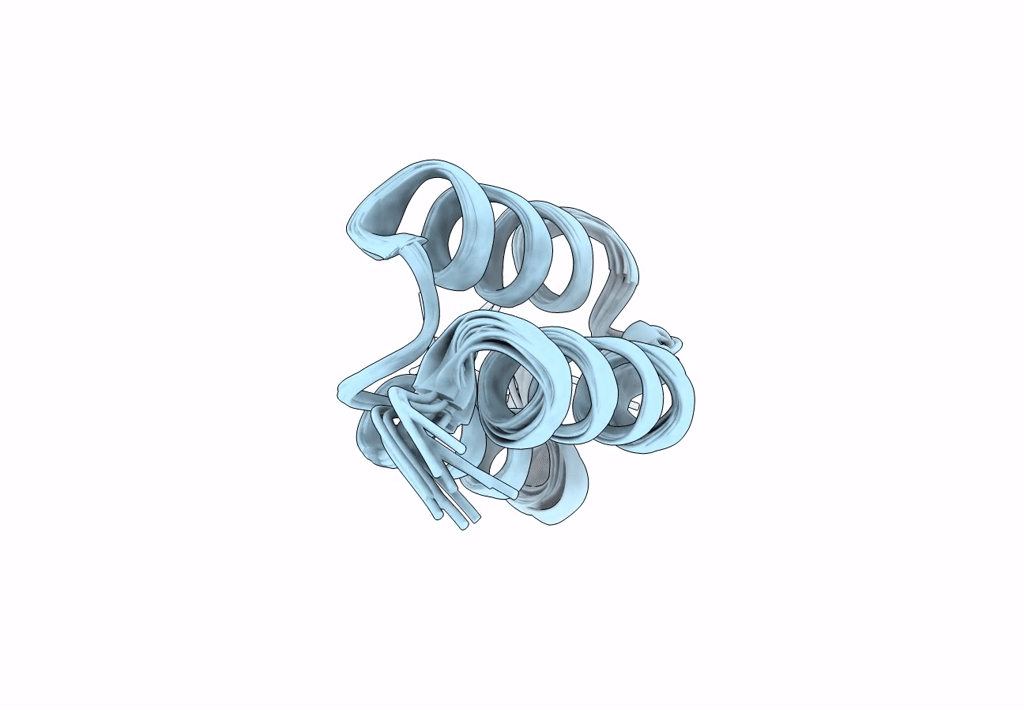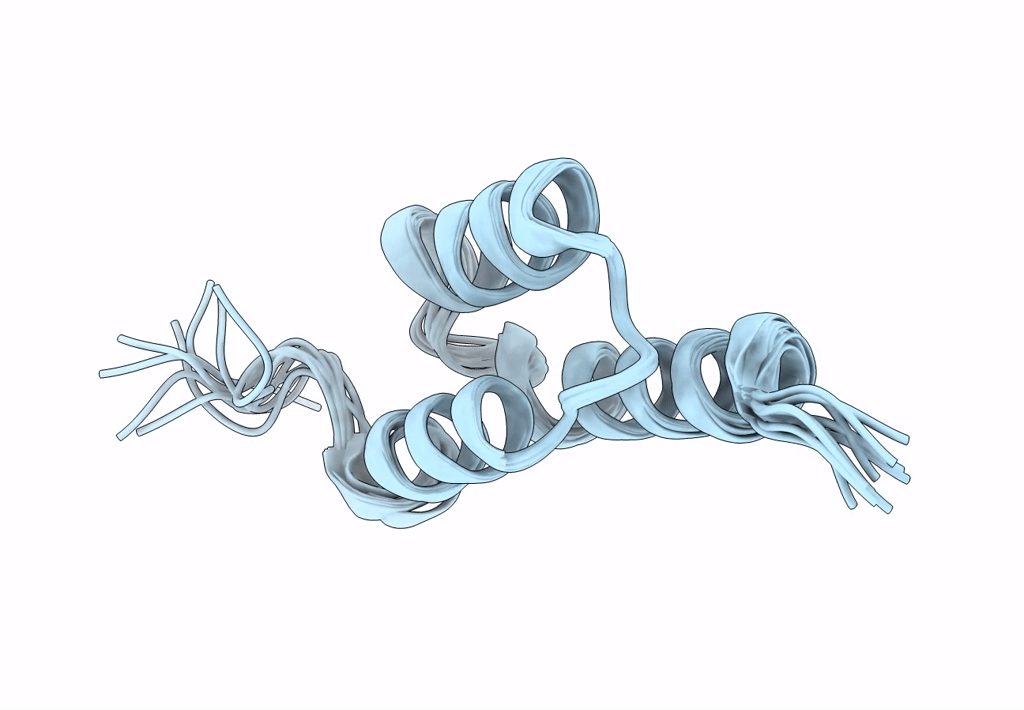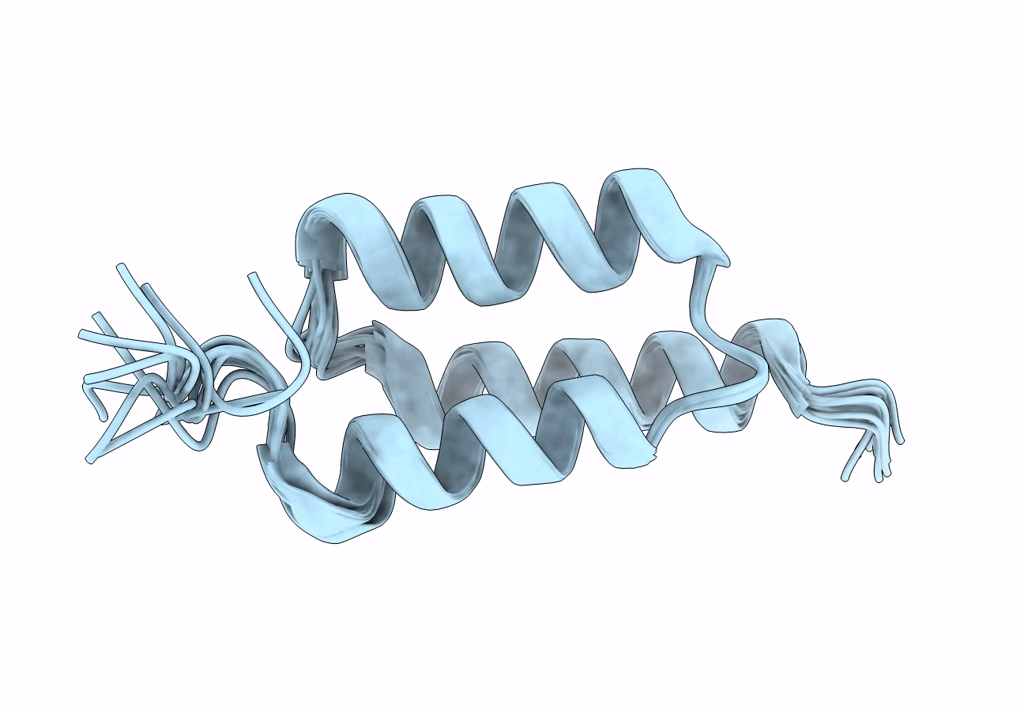
Title:
Backbone-modified variant of the B domain of Staphylococcal protein A: beta3- and ACPC-residues in helix 2
Biological Source:
Source Organism(s):
Staphylococcus aureus (Taxon ID: 1280)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy


