Deposition Date
2022-02-18
Release Date
2022-08-10
Last Version Date
2024-10-30
Entry Detail
PDB ID:
7U0P
Keywords:
Title:
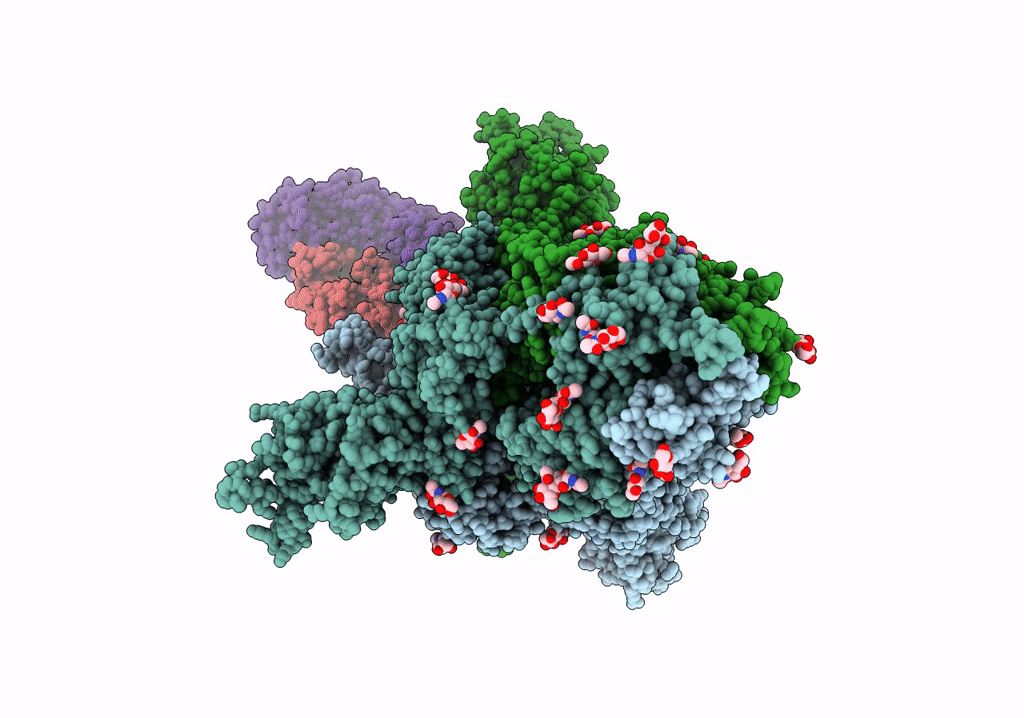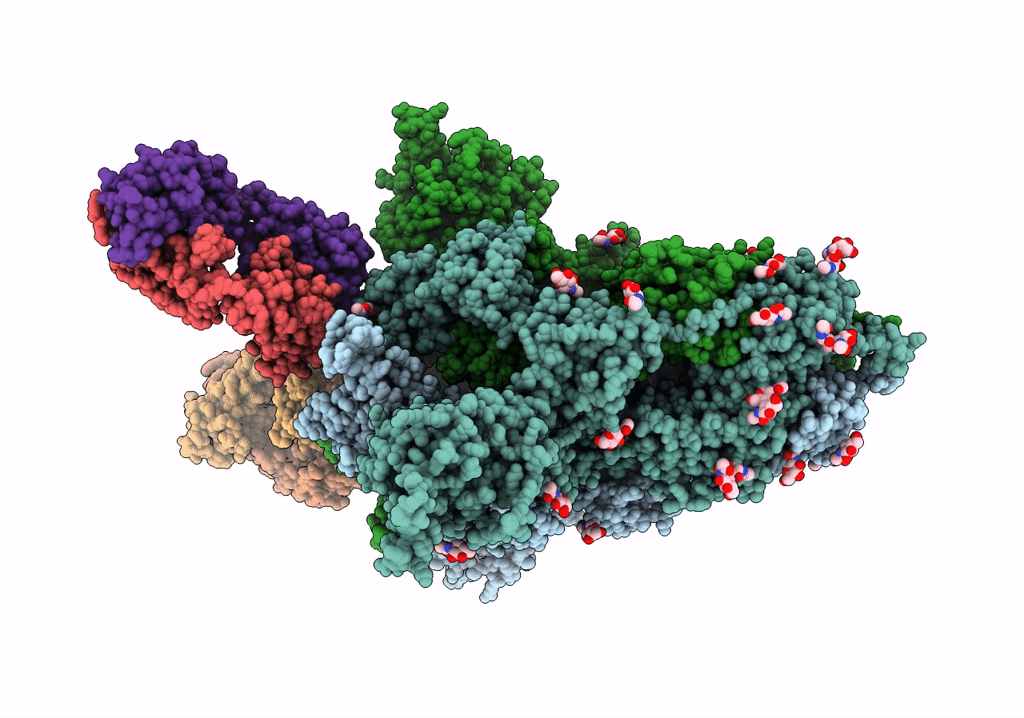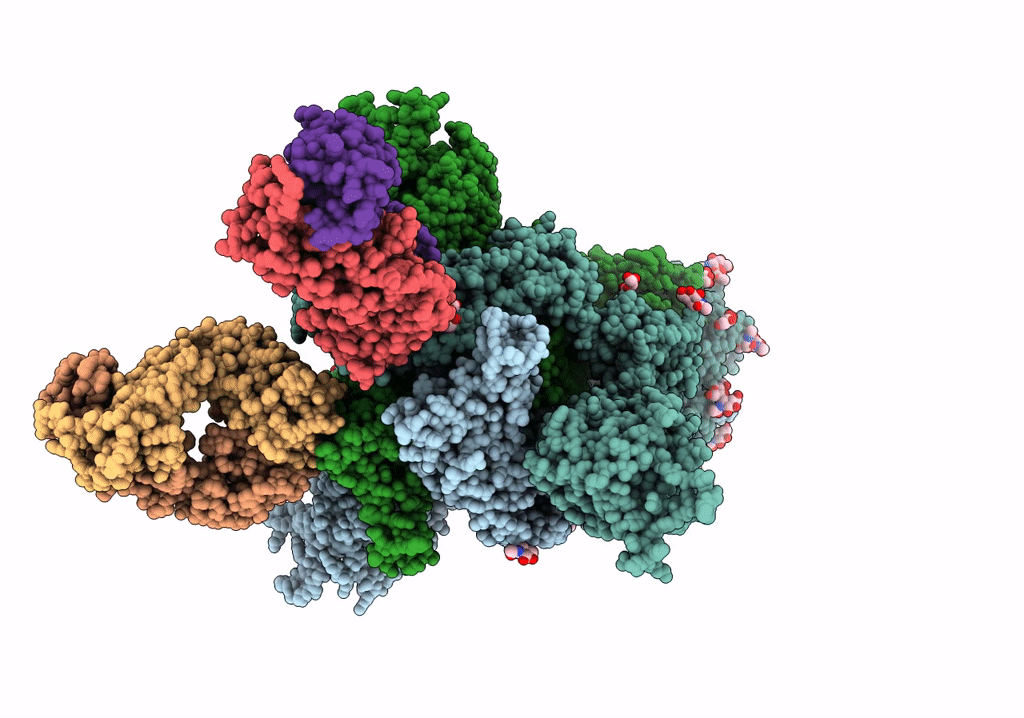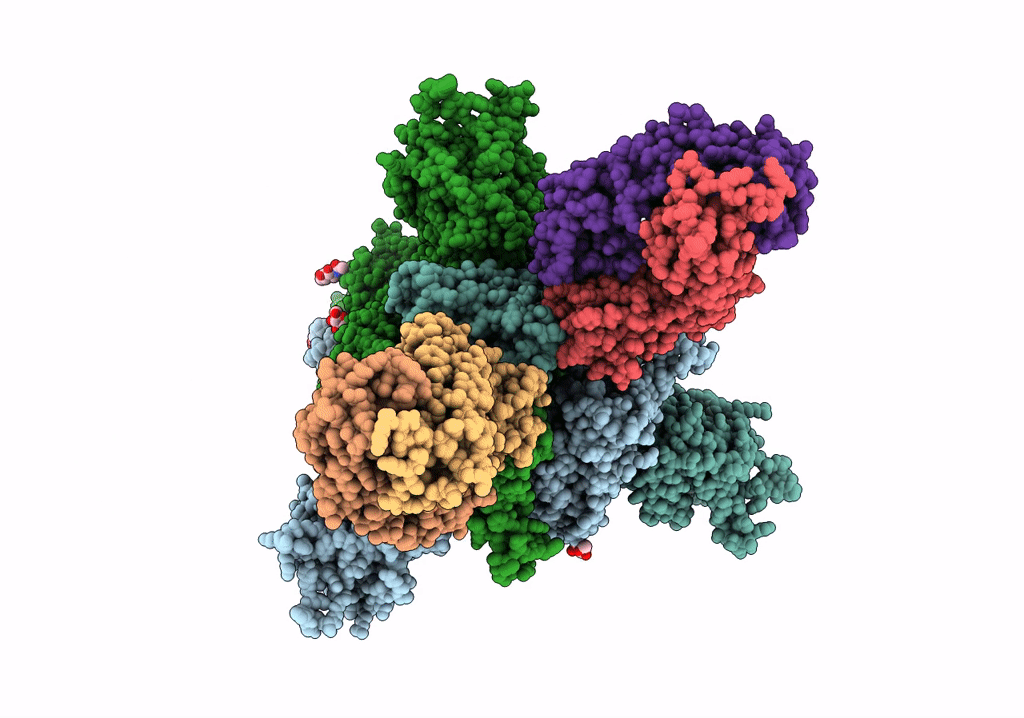
SARS-Cov2 S protein structure in complex with neutralizing monoclonal antibody 002-S21F2
Biological Source:
Source Organism(s):
Severe acute respiratory syndrome coronavirus 2 (Taxon ID: 2697049)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.76 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


