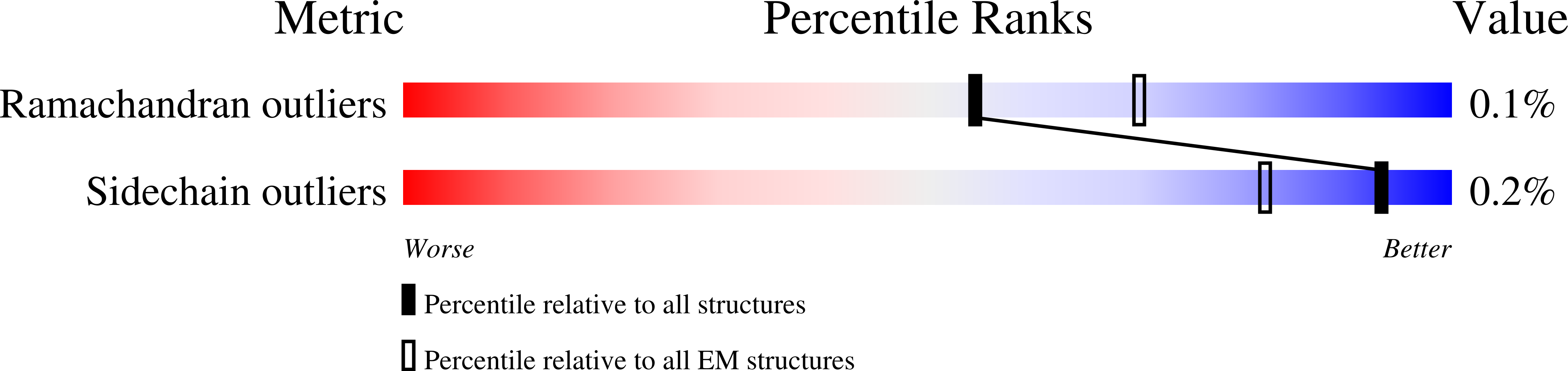
Deposition Date
2022-02-17
Release Date
2022-04-27
Last Version Date
2024-11-13
Entry Detail
PDB ID:
7U06
Keywords:
Title:
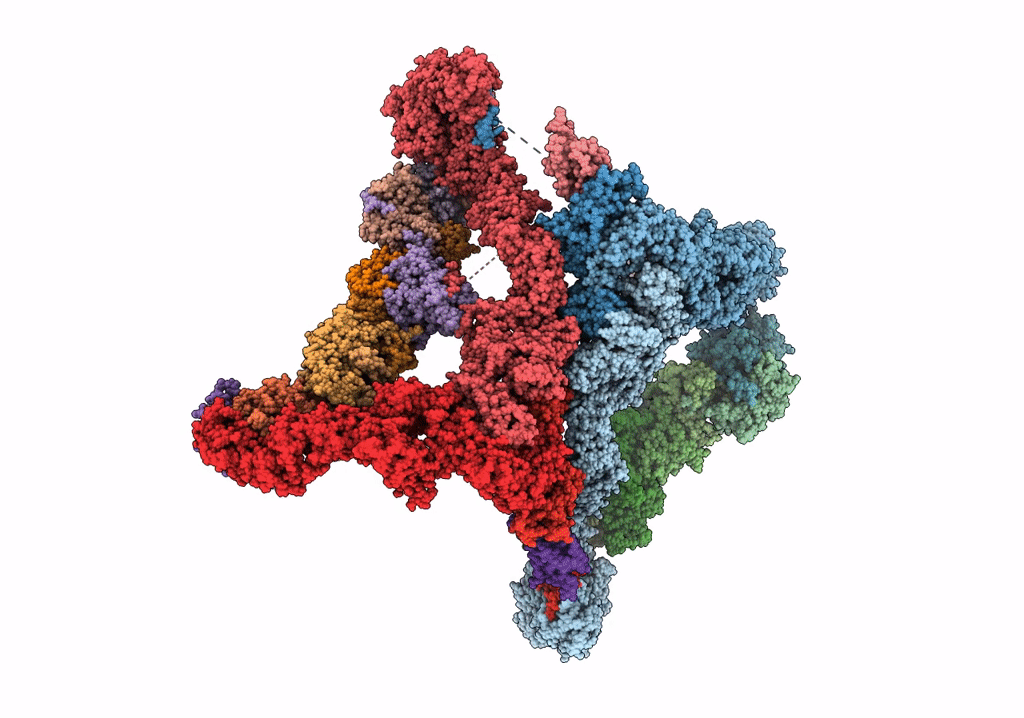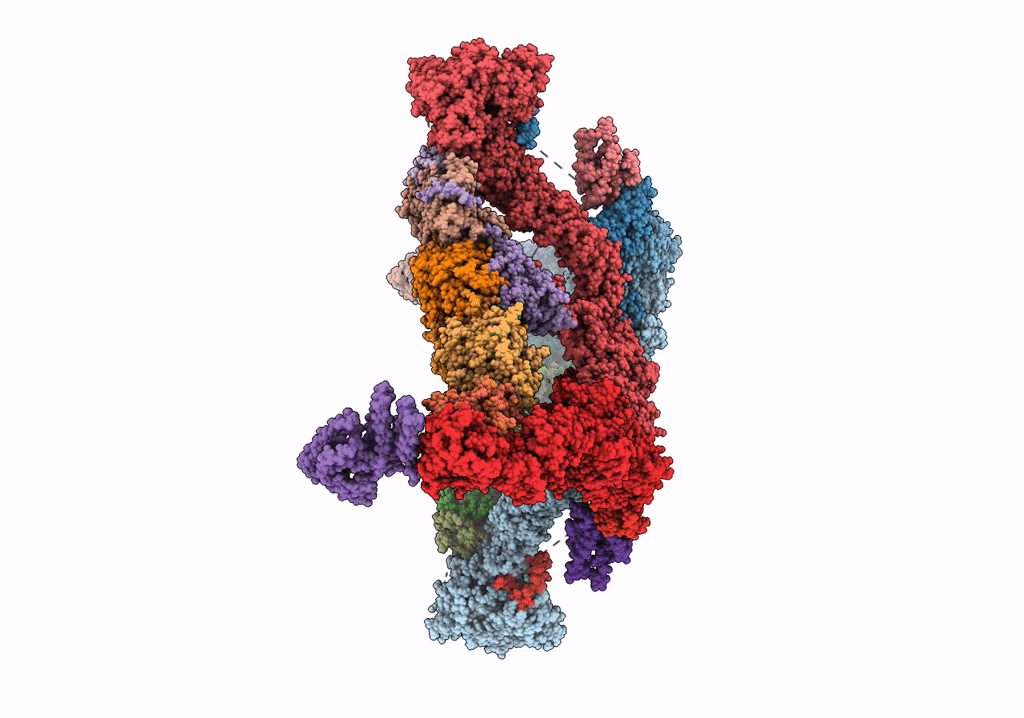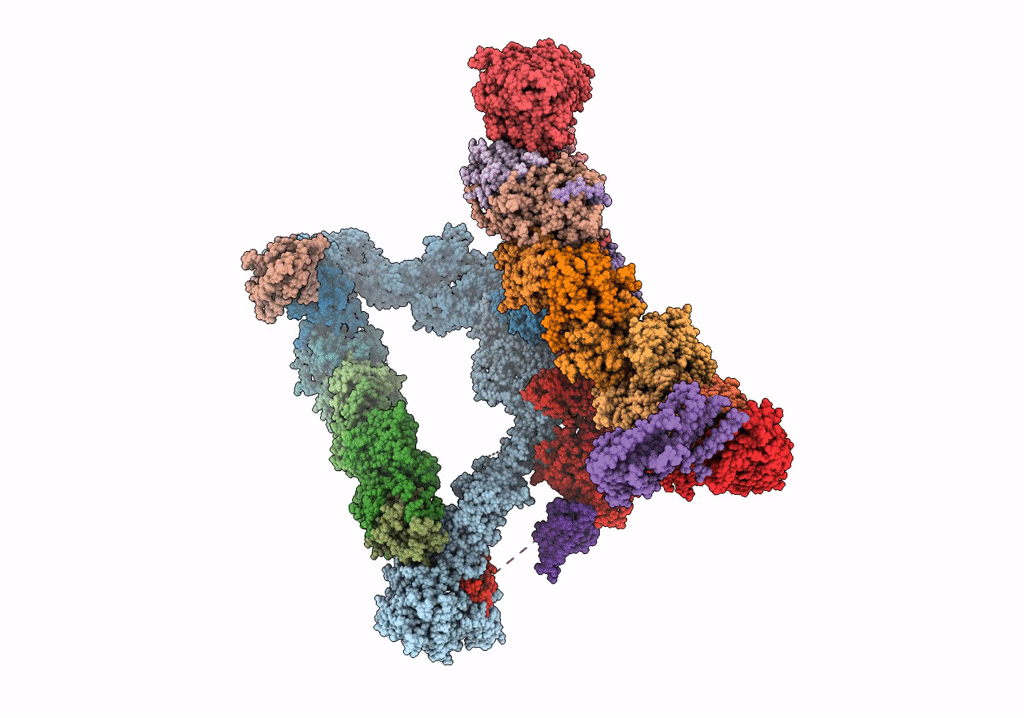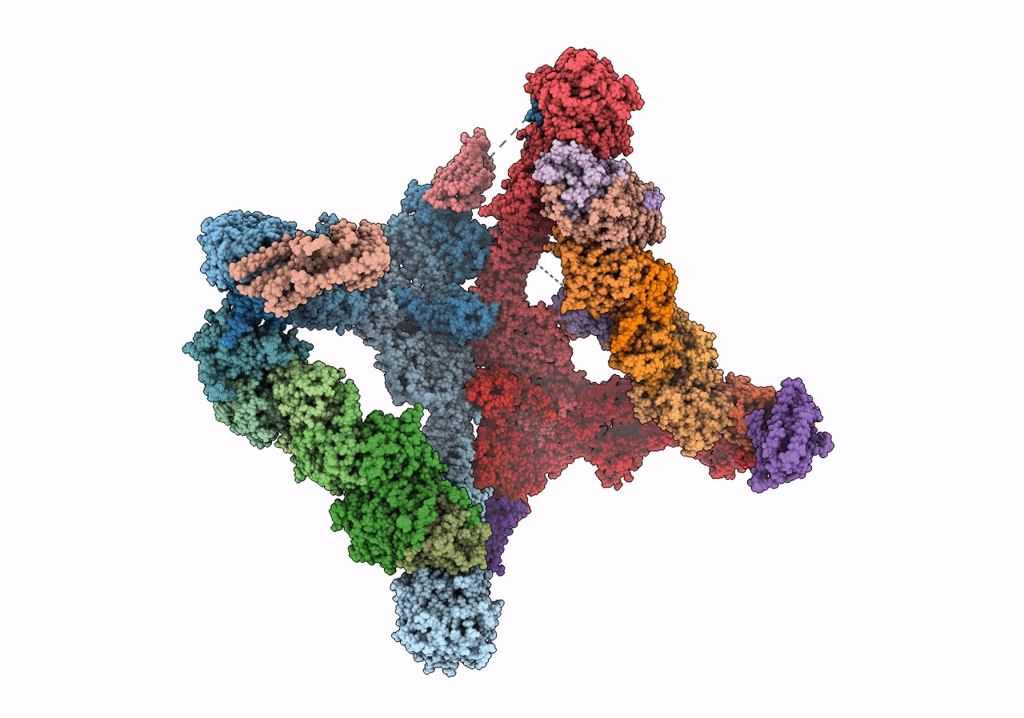
Structure of the yeast TRAPPII-Rab11/Ypt32 complex in the closed/open state (composite structure)
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 4932)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.20 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE