Deposition Date
2022-02-03
Release Date
2022-09-28
Last Version Date
2023-10-25
Entry Detail
PDB ID:
7TUR
Keywords:
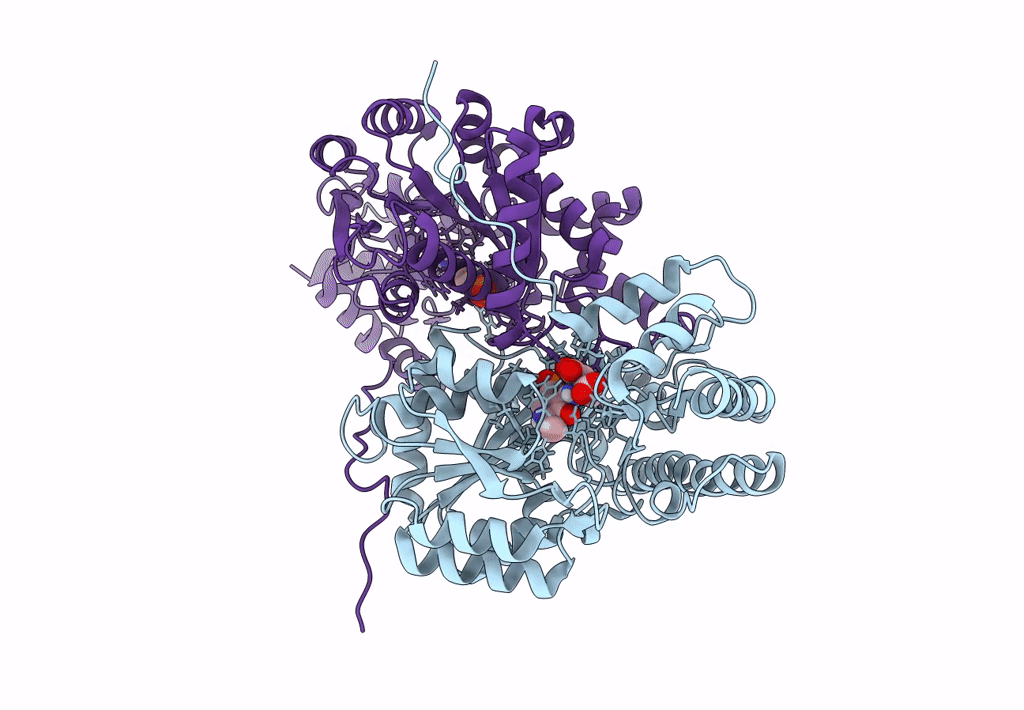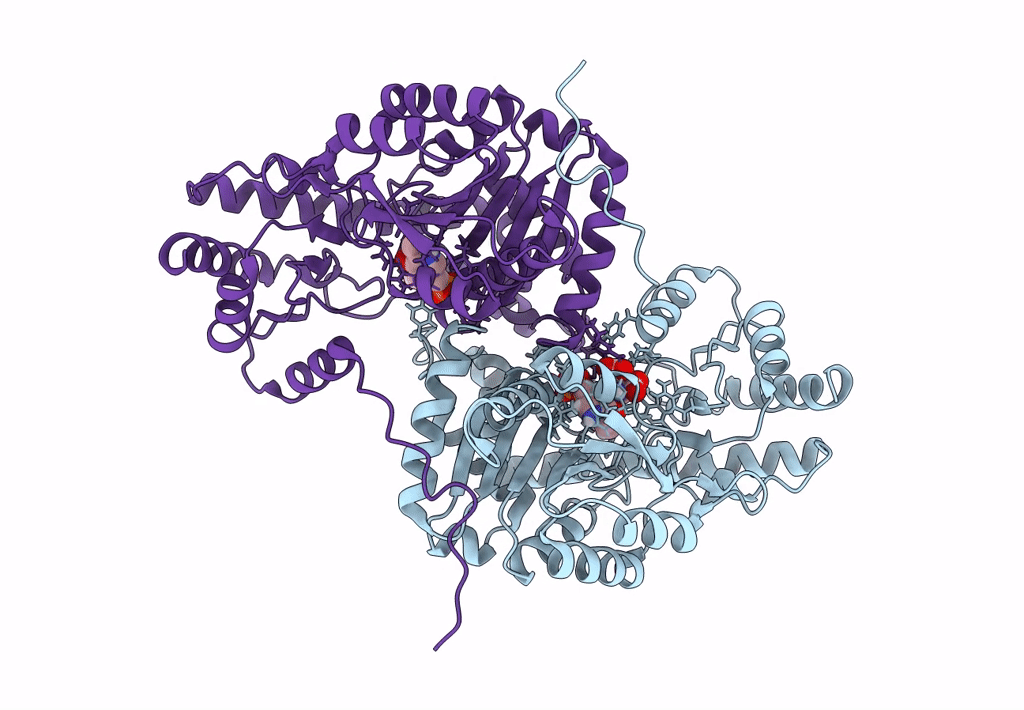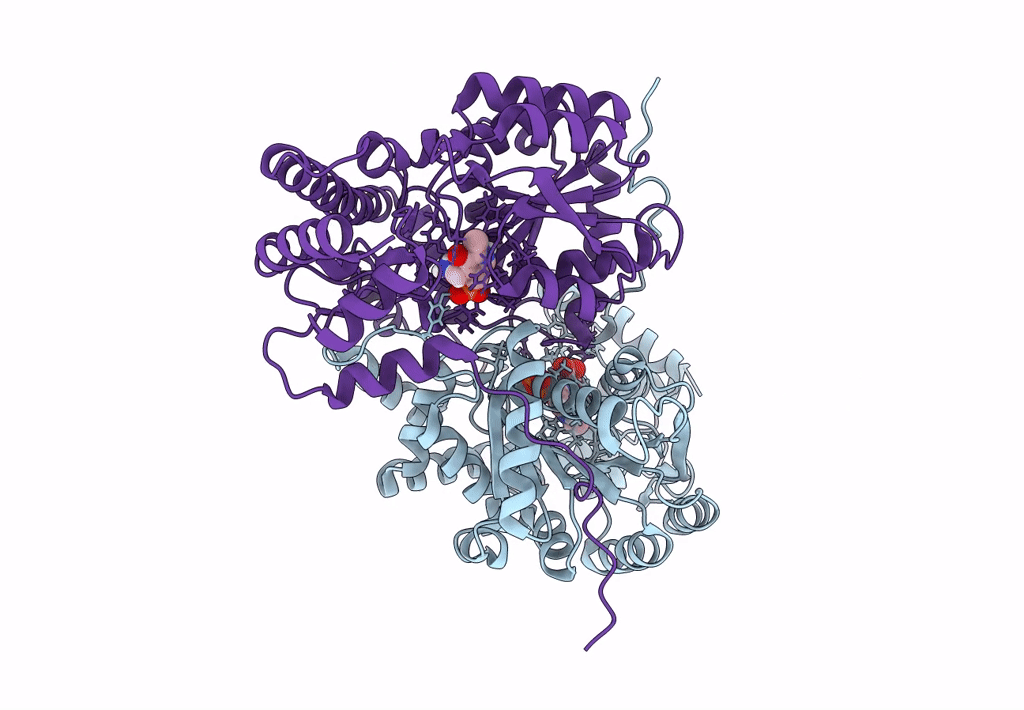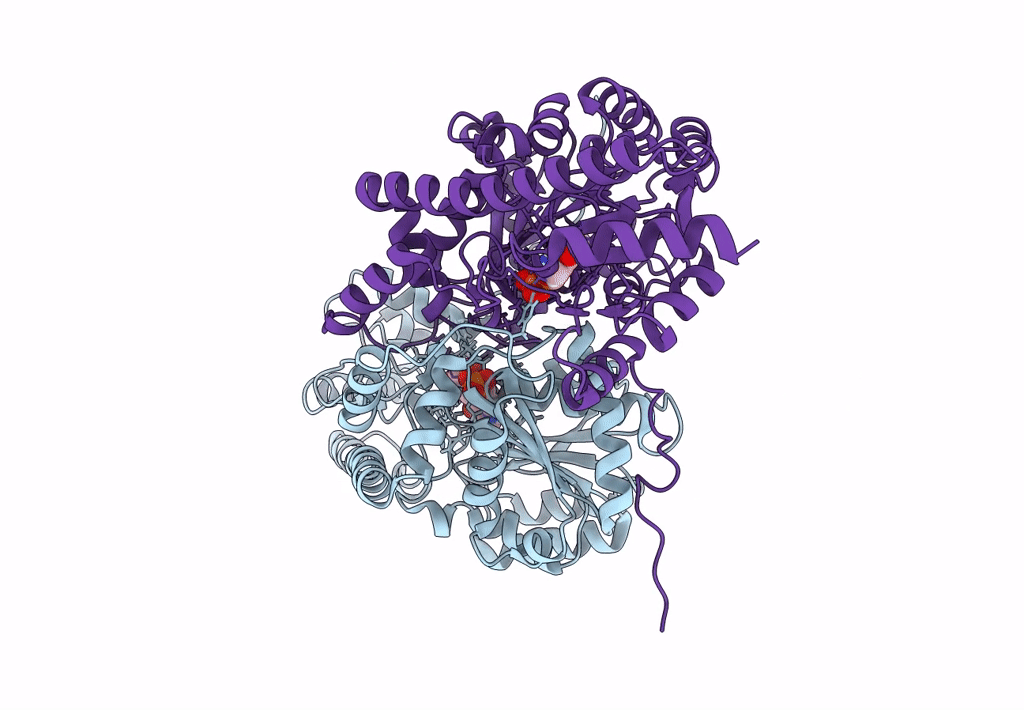
Title:
Joint X-ray/neutron structure of aspastate aminotransferase (AAT) in complex with pyridoxamine 5'-phosphate (PMP)
Biological Source:
Source Organism(s):
Sus scrofa (Taxon ID: 9823)
Expression System(s):
Method Details:
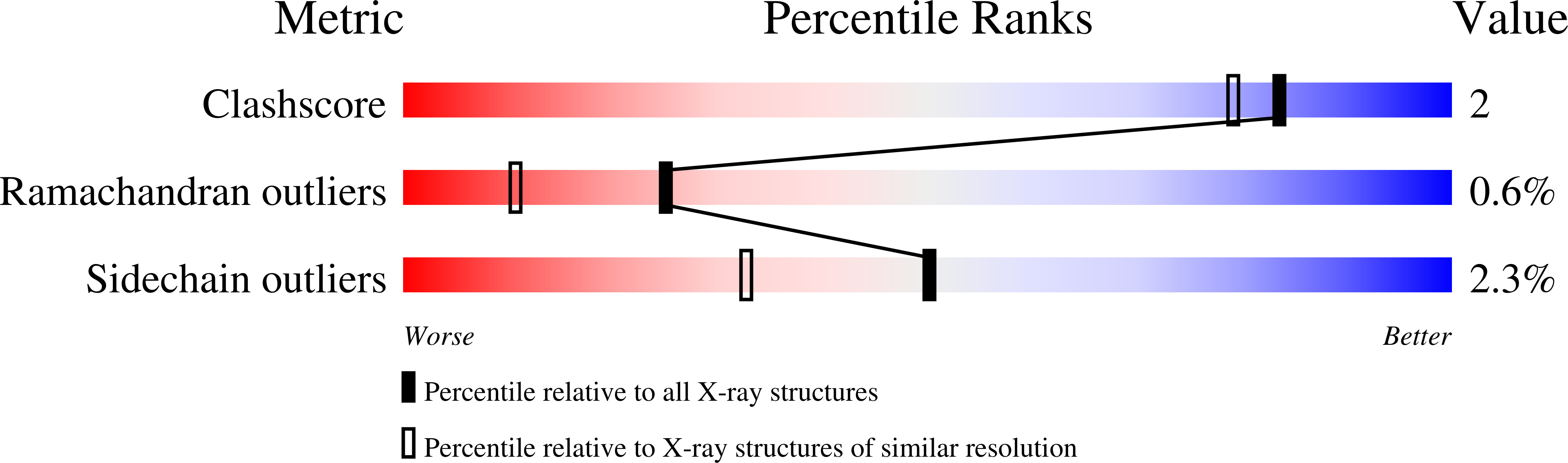
Experimental Method:
R-Value Free:
['0.23
R-Value Work:
['0.20
R-Value Observed:
['?', '?'].00
Space Group:
P 21 21 21