Deposition Date
2022-01-31
Release Date
2022-07-13
Last Version Date
2023-10-18
Entry Detail
PDB ID:
7TSA
Keywords:
Title:
Structure of rat neuronal nitric oxide synthase R349A/H692F mutant heme domain in complex with 4-methyl-6-(3-((methylamino)methyl)phenyl)pyridin-2-amine
Biological Source:
Source Organism(s):
Rattus norvegicus (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
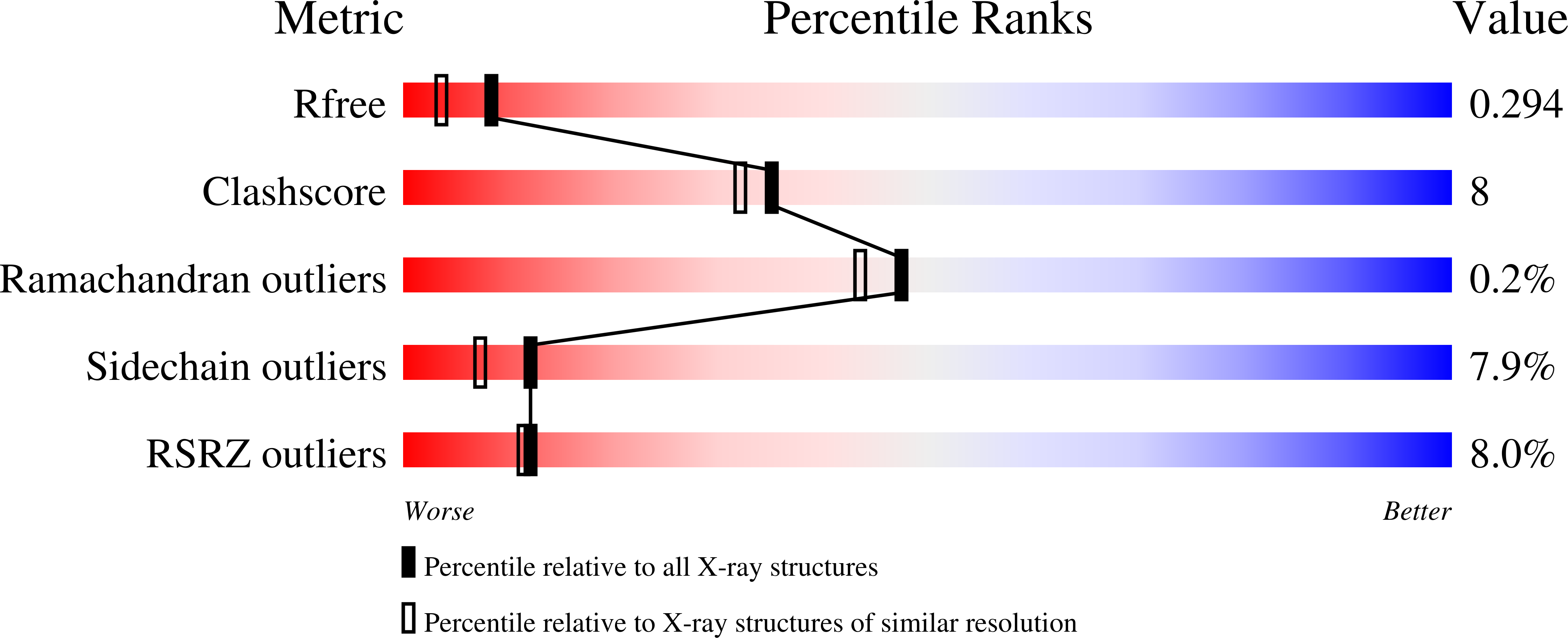
Resolution:
2.03 Å
R-Value Free:
0.29
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
C 2 2 21