Deposition Date
2022-01-23
Release Date
2022-03-02
Last Version Date
2023-10-18
Entry Detail
PDB ID:
7TO5
Keywords:
Title:
HIV-1 wild type protease with GRL-05816A, with C-4 substituted cyclohexane-fused bis-tetrahydrofuran (Chf-THF) derivatives as P2-ligand [diastereomer 1]
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Expression System(s):
Method Details:
Experimental Method:
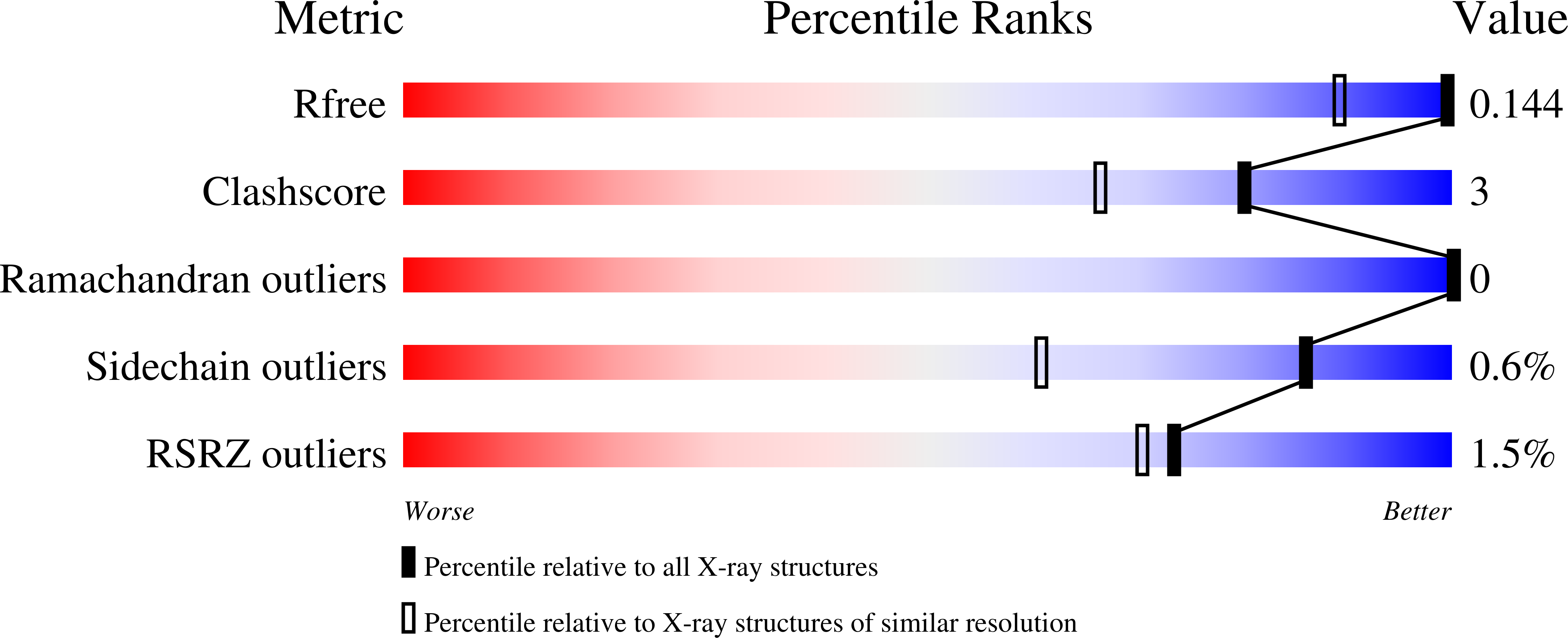
Resolution:
1.13 Å
R-Value Free:
0.14
R-Value Work:
0.12
R-Value Observed:
0.12
Space Group:
P 21 21 2