Deposition Date
1983-01-27
Release Date
1983-03-09
Last Version Date
2024-10-23
Entry Detail
PDB ID:
7TLN
Keywords:
Title:
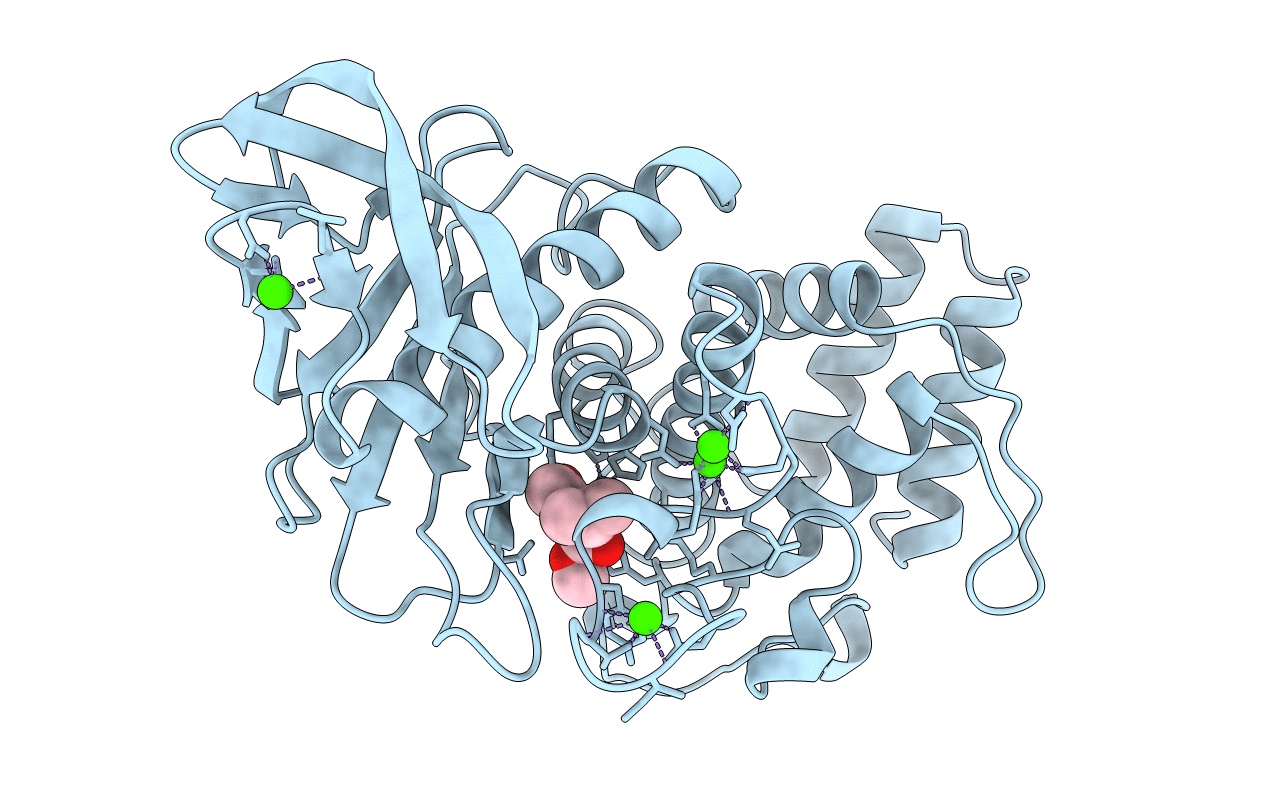
STRUCTURAL ANALYSIS OF THE INHIBITION OF THERMOLYSIN BY AN ACTIVE-SITE-DIRECTED IRREVERSIBLE INHIBITOR
Biological Source:
Source Organism(s):
Bacillus thermoproteolyticus (Taxon ID: 1427)
Method Details: