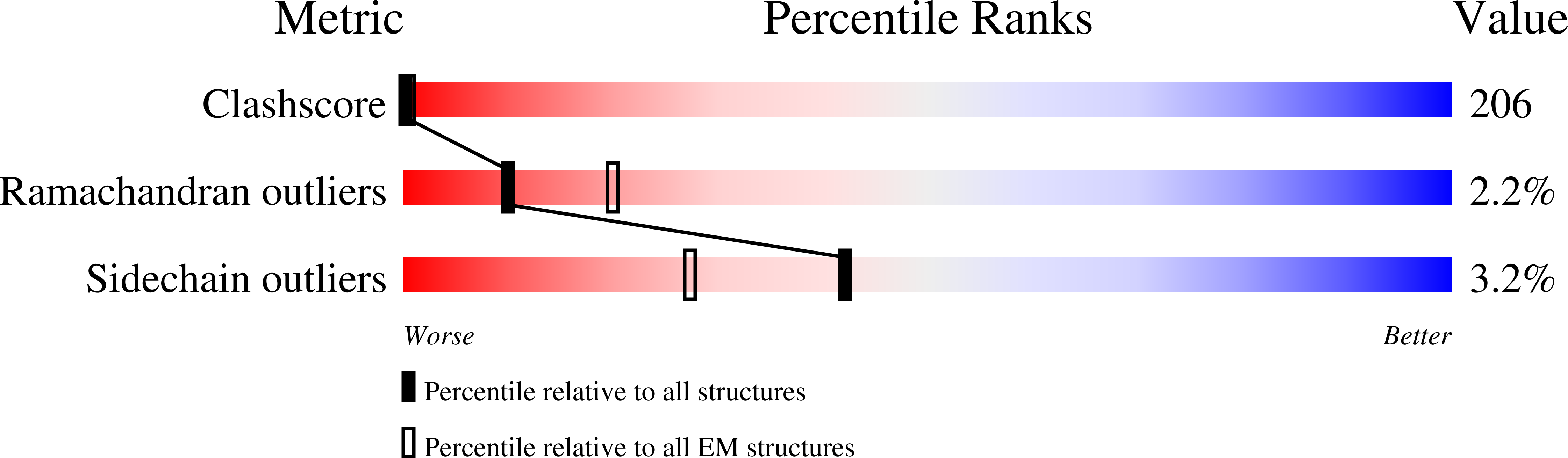
Deposition Date
2022-01-14
Release Date
2022-11-23
Last Version Date
2024-06-05
Entry Detail
PDB ID:
7TJ7
Keywords:
Title:
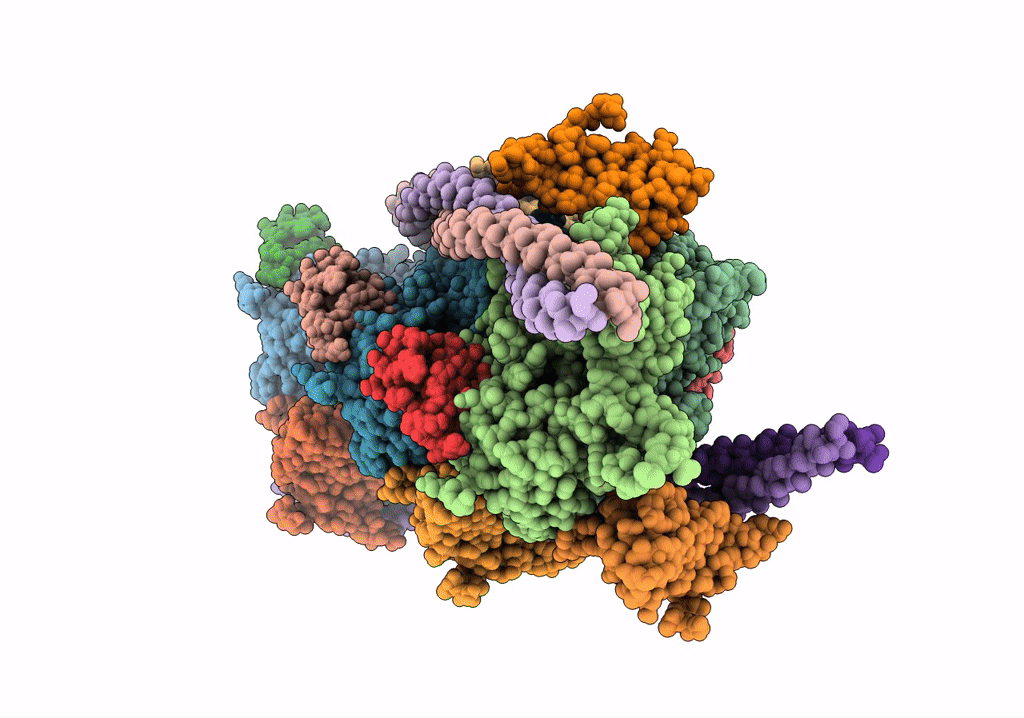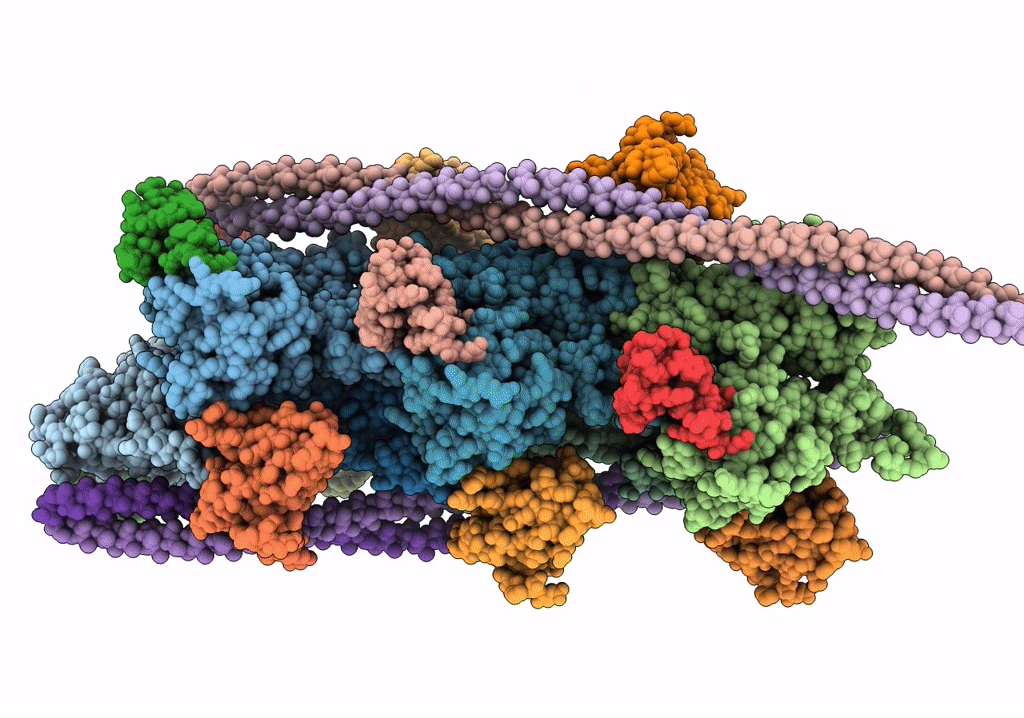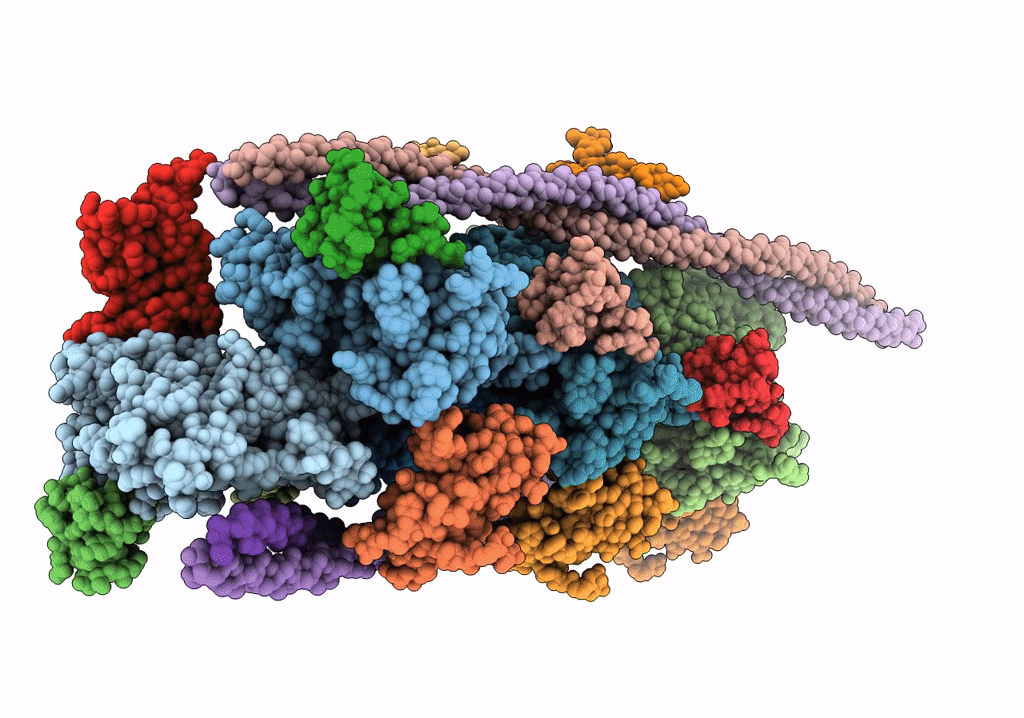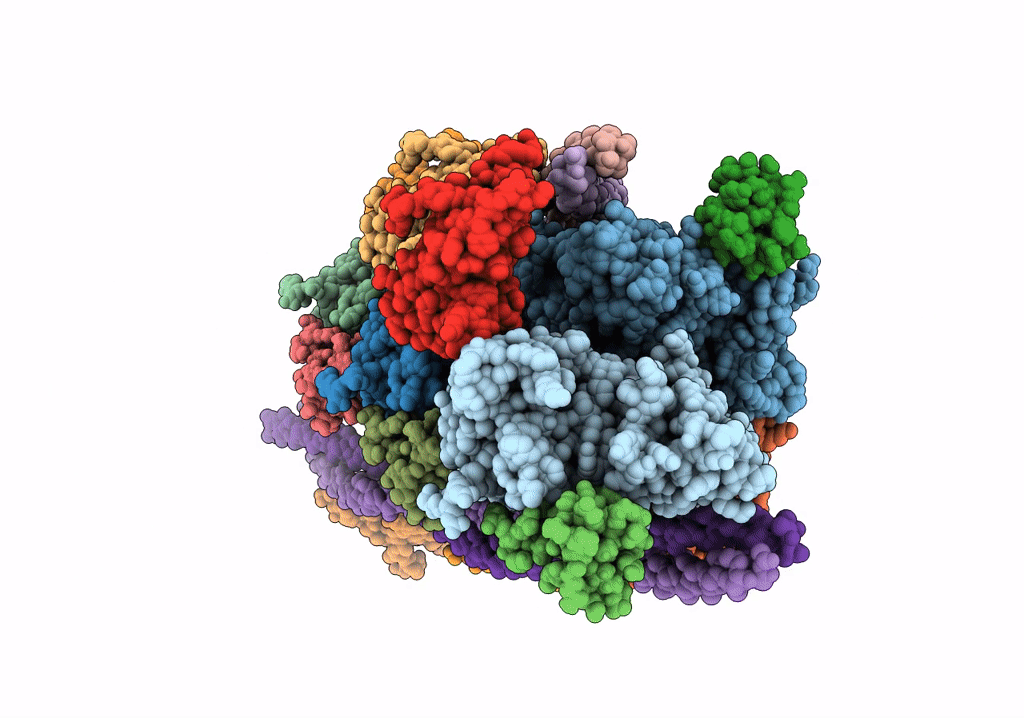
Cardiac thin filament decorated with C1 Ig-domain and regulatory M-domain of cardiac myosin binding protein C (cMyBP-C)
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Sus scrofa (Taxon ID: 9823)
Sus scrofa (Taxon ID: 9823)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
8.00 Å
Aggregation State:
HELICAL ARRAY
Reconstruction Method:
HELICAL