Deposition Date
2021-12-28
Release Date
2022-03-23
Last Version Date
2024-02-28
Entry Detail
PDB ID:
7TCR
Keywords:
Title:
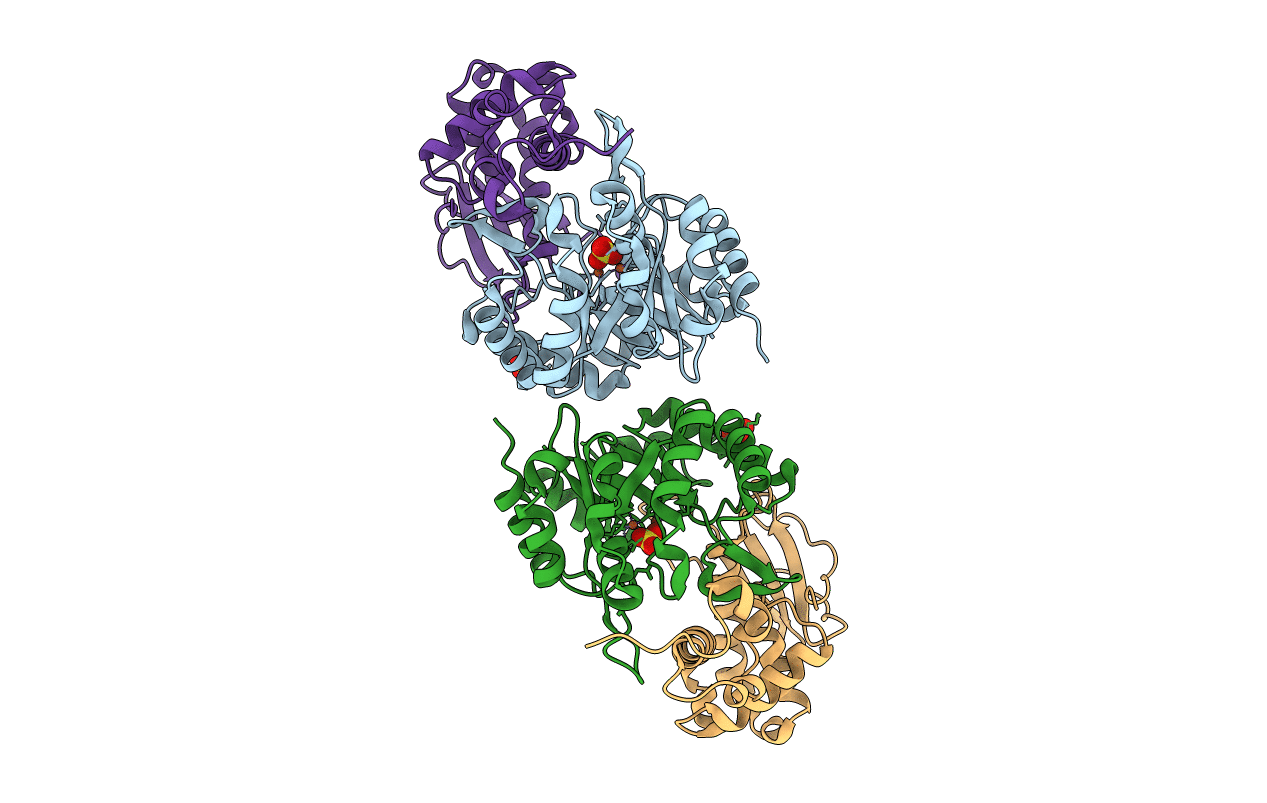
Methanobactin biosynthetic protein complex of MbnB and MbnC from Methylosinus trichosporium OB3b at 2.62 Angstrom resolution
Biological Source:
Source Organism(s):
Methylosinus trichosporium OB3b (Taxon ID: 595536)
Expression System(s):
Method Details:
Experimental Method:
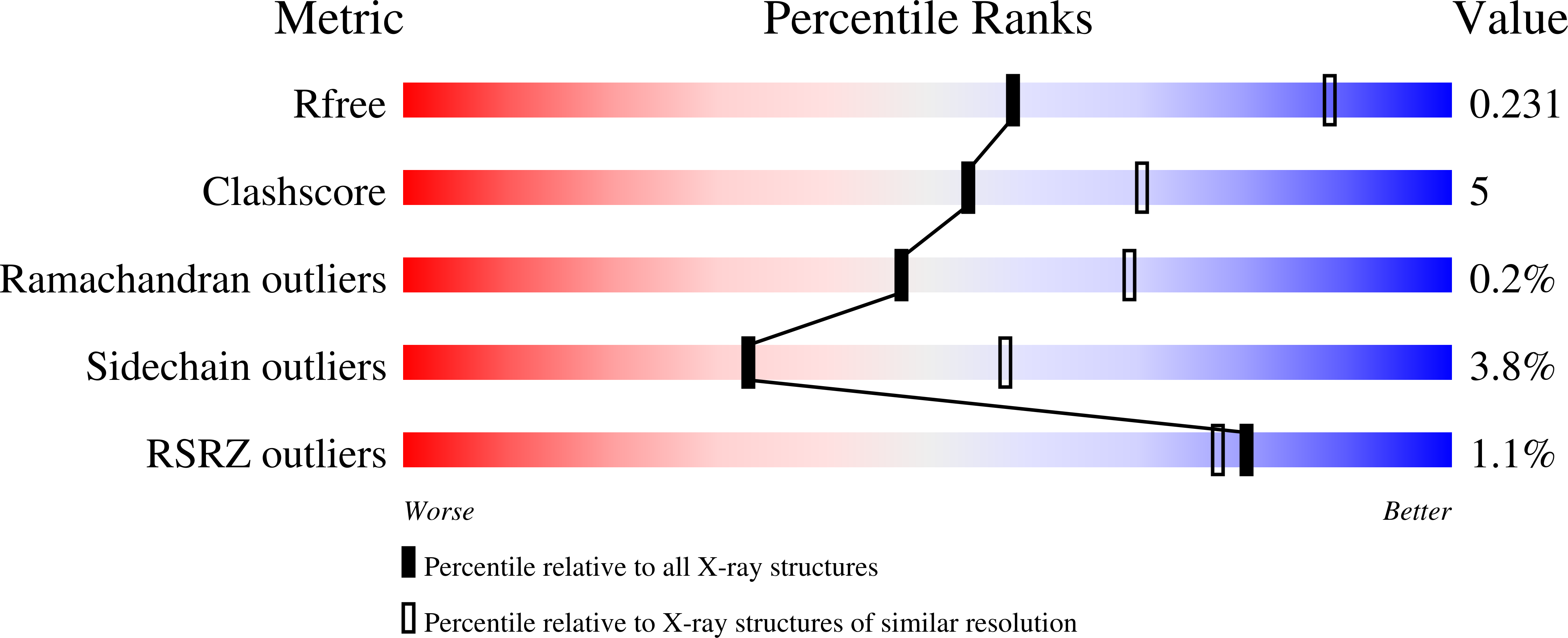
Resolution:
2.62 Å
R-Value Free:
0.23
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
C 2 2 21