Deposition Date
2021-11-19
Release Date
2022-04-06
Last Version Date
2025-05-28
Entry Detail
PDB ID:
7SWD
Keywords:
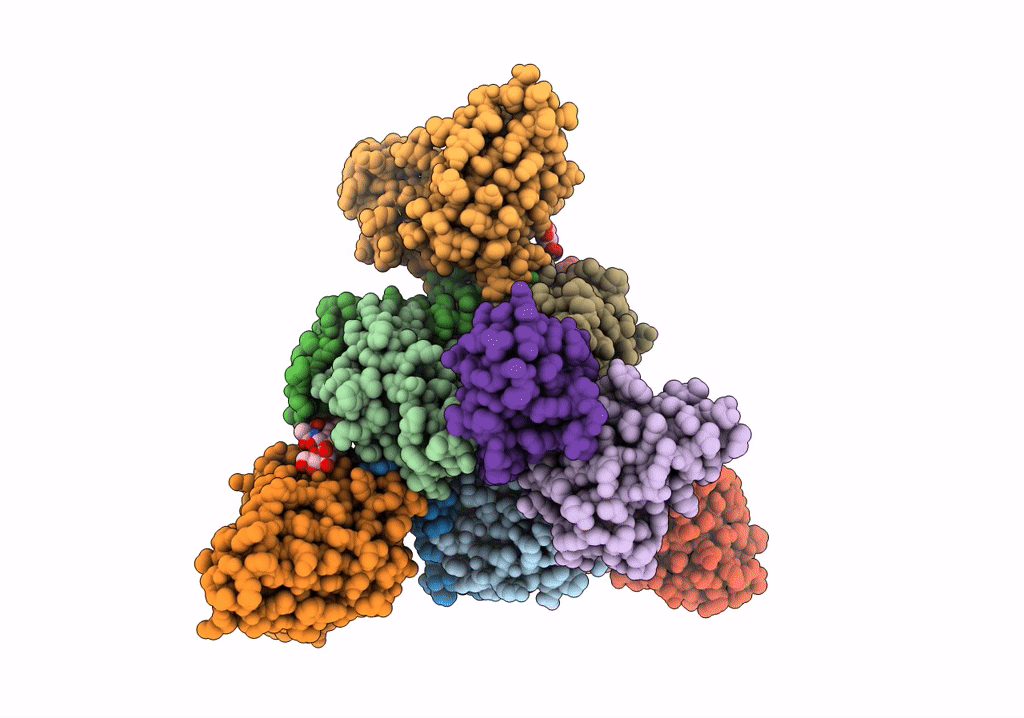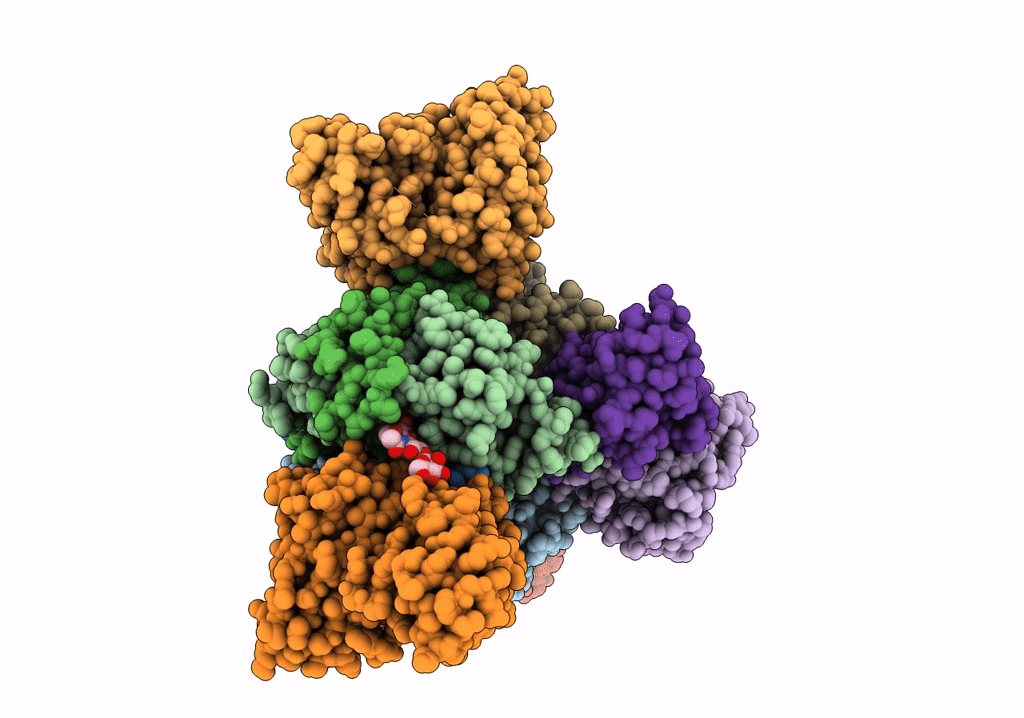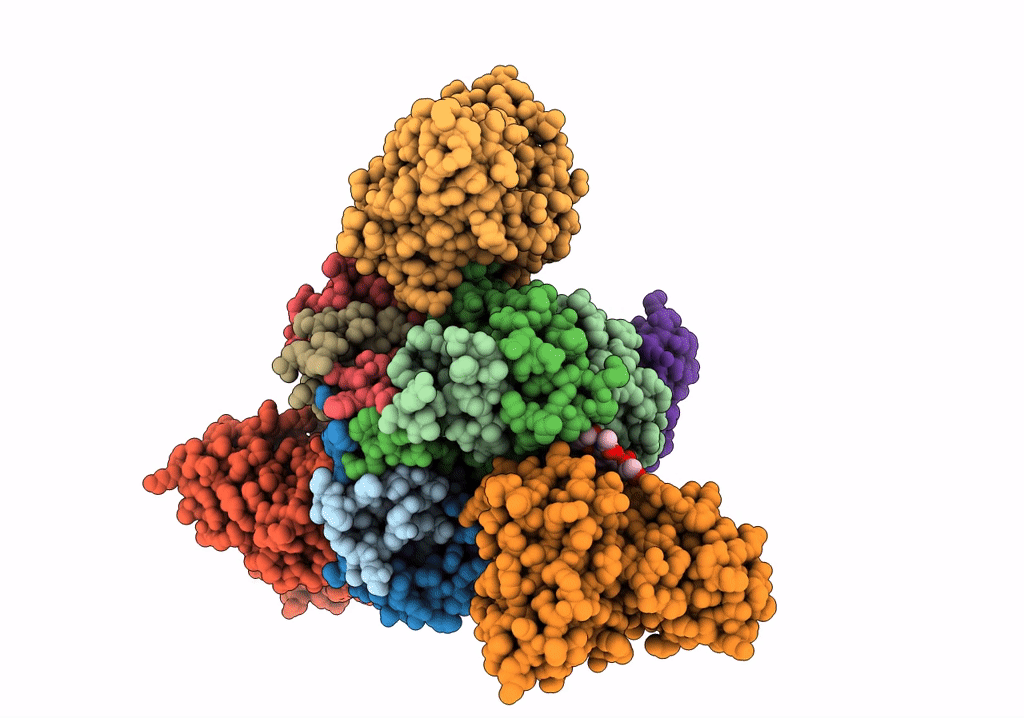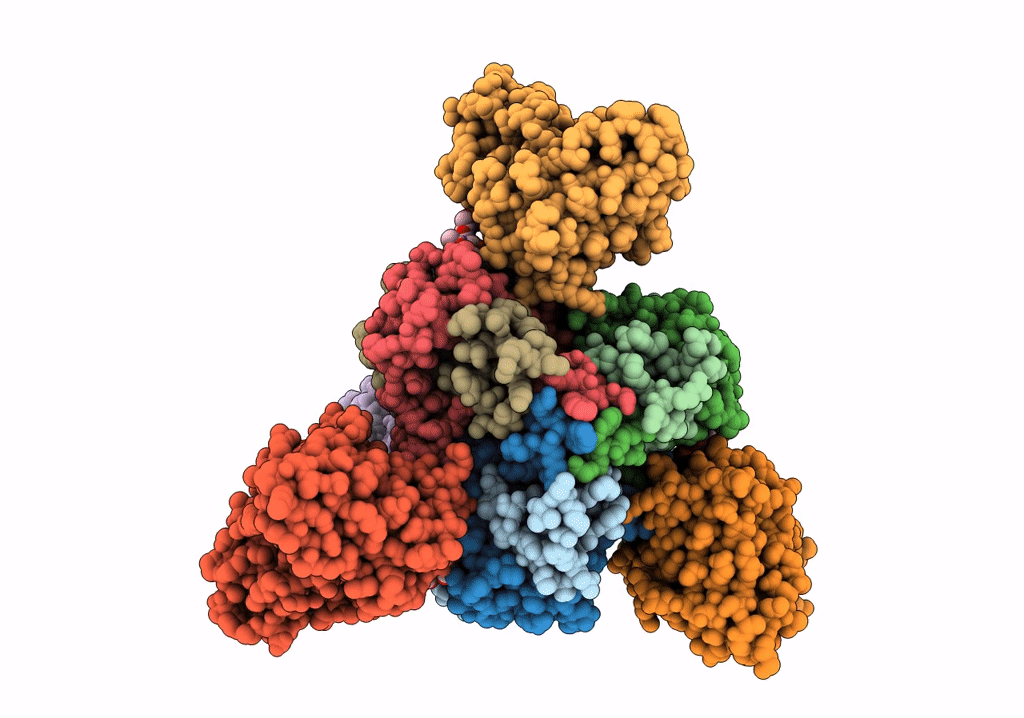
Title:
Structure of EBOV GP lacking the mucin-like domain with 1C11 scFv and 1C3 Fab bound
Biological Source:
Source Organism(s):
Ebola virus - Gabon (1994-1997) (Taxon ID: 128947)
Zaire ebolavirus (Taxon ID: 186538)
Homo sapiens (Taxon ID: 9606)
Zaire ebolavirus (Taxon ID: 186538)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.59 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


