Deposition Date
2021-09-28
Release Date
2022-08-24
Last Version Date
2024-11-20
Entry Detail
PDB ID:
7SCO
Keywords:
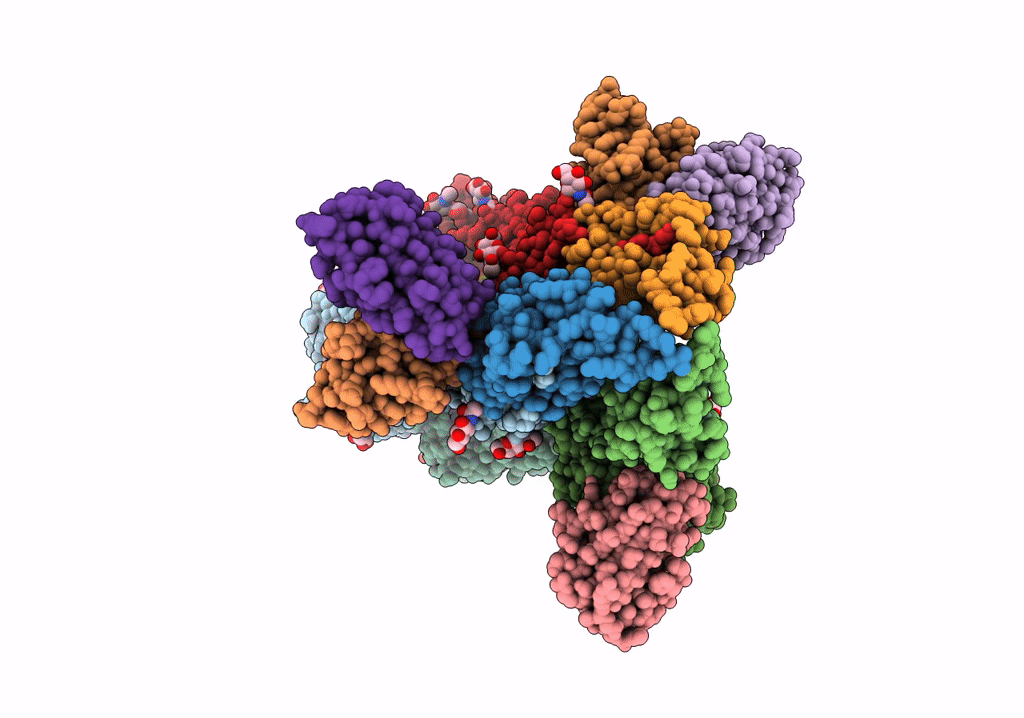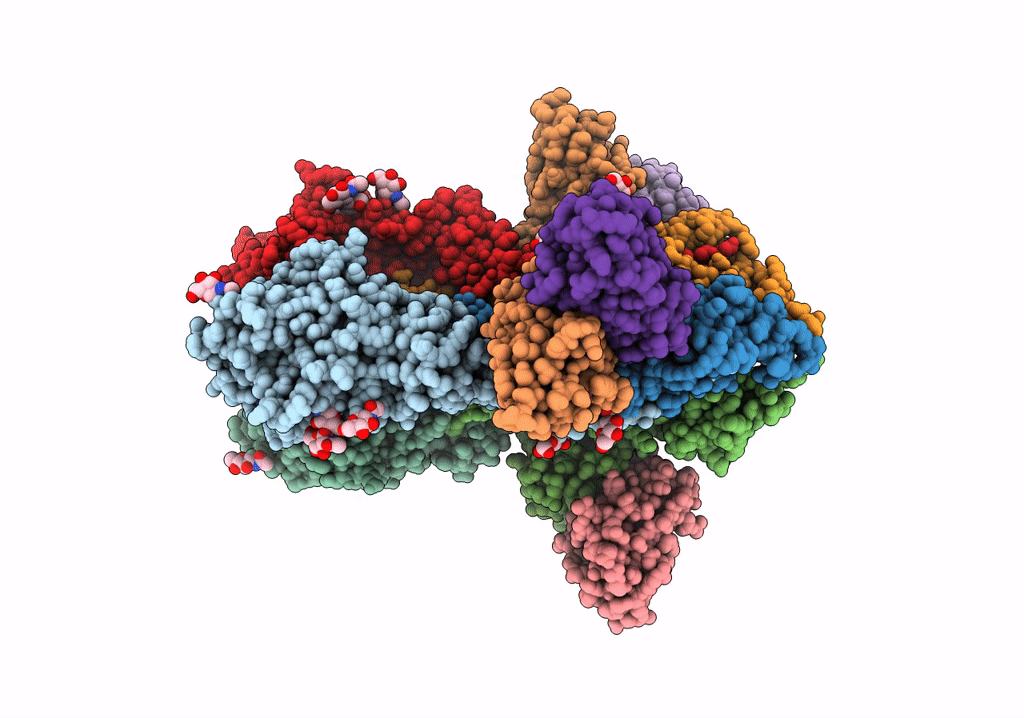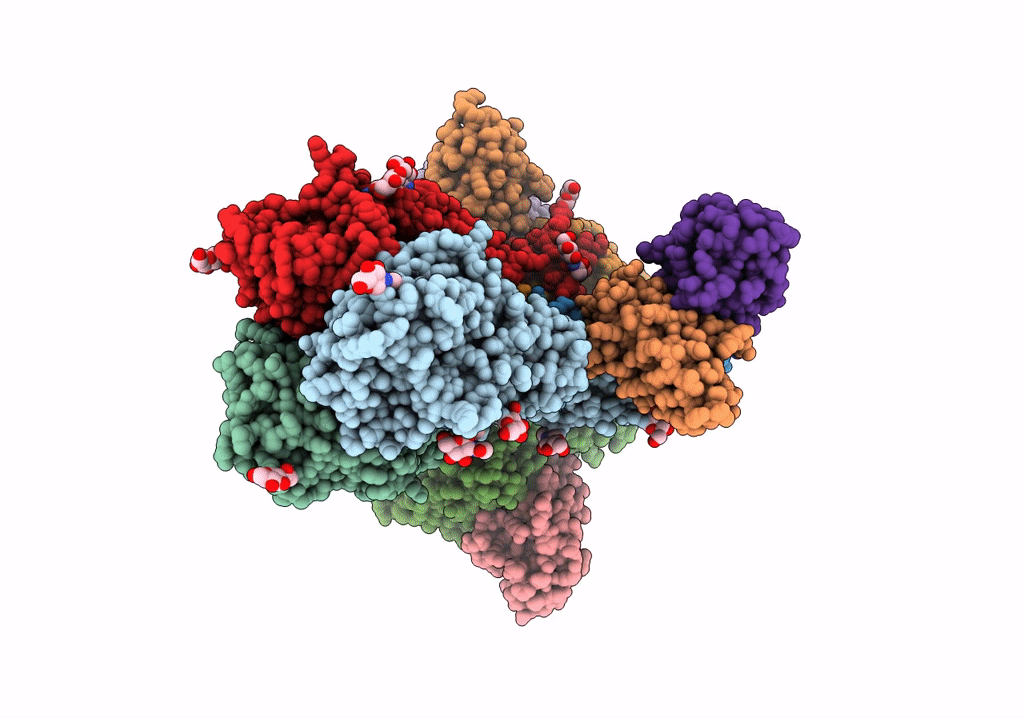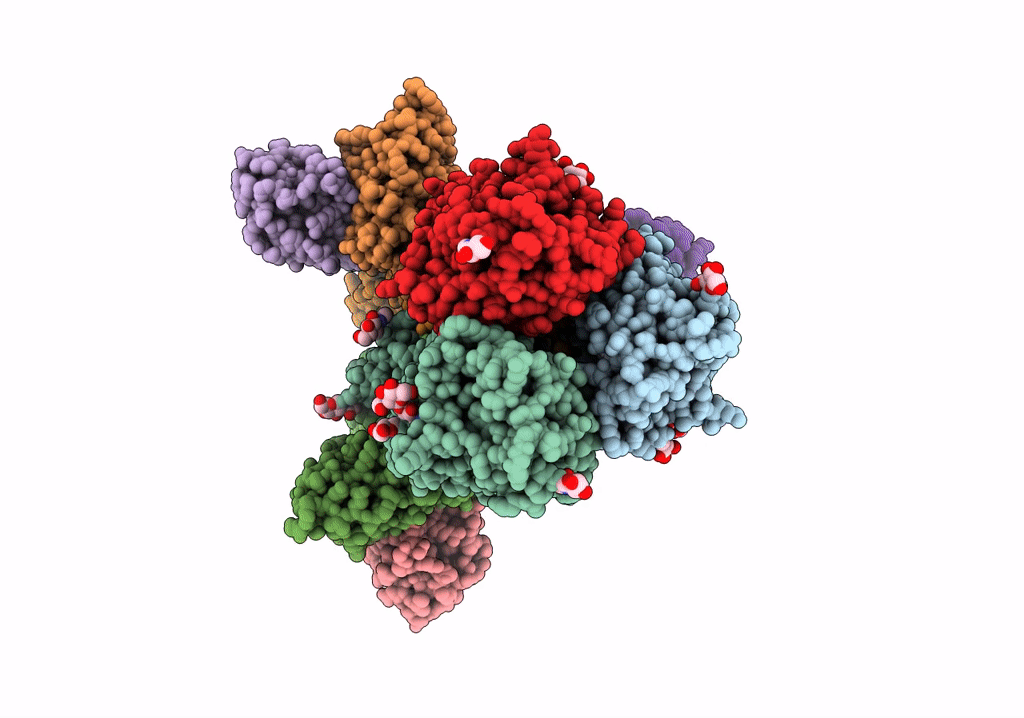
Title:
Structure of H1 influenza hemagglutinin bound to Fab 310-39G10
Biological Source:
Source Organism(s):
Influenza A virus (strain A/New Zealand:South Canterbury/35/2000 H1N1) (Taxon ID: 363066)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.37 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


