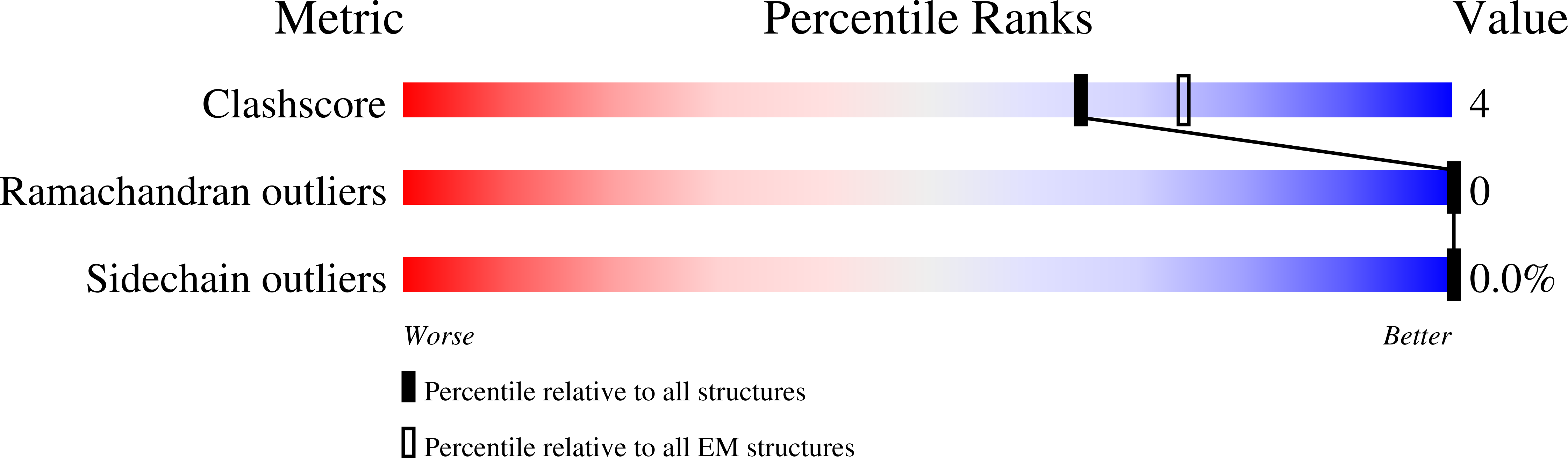
Deposition Date
2021-09-13
Release Date
2021-11-03
Last Version Date
2021-11-17
Entry Detail
PDB ID:
7S6C
Keywords:
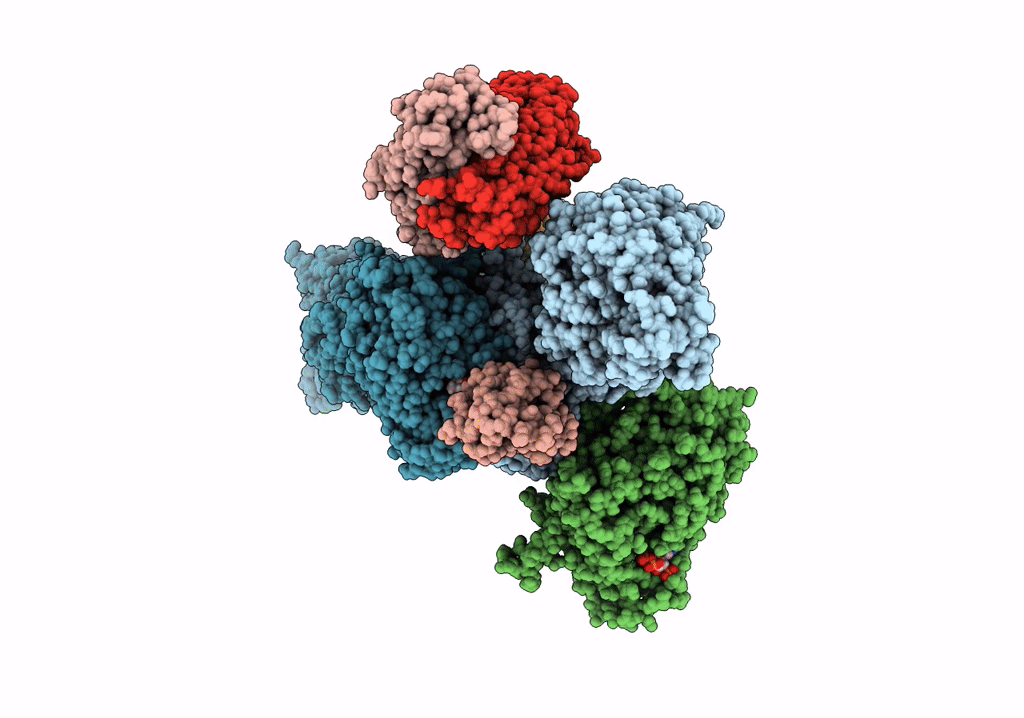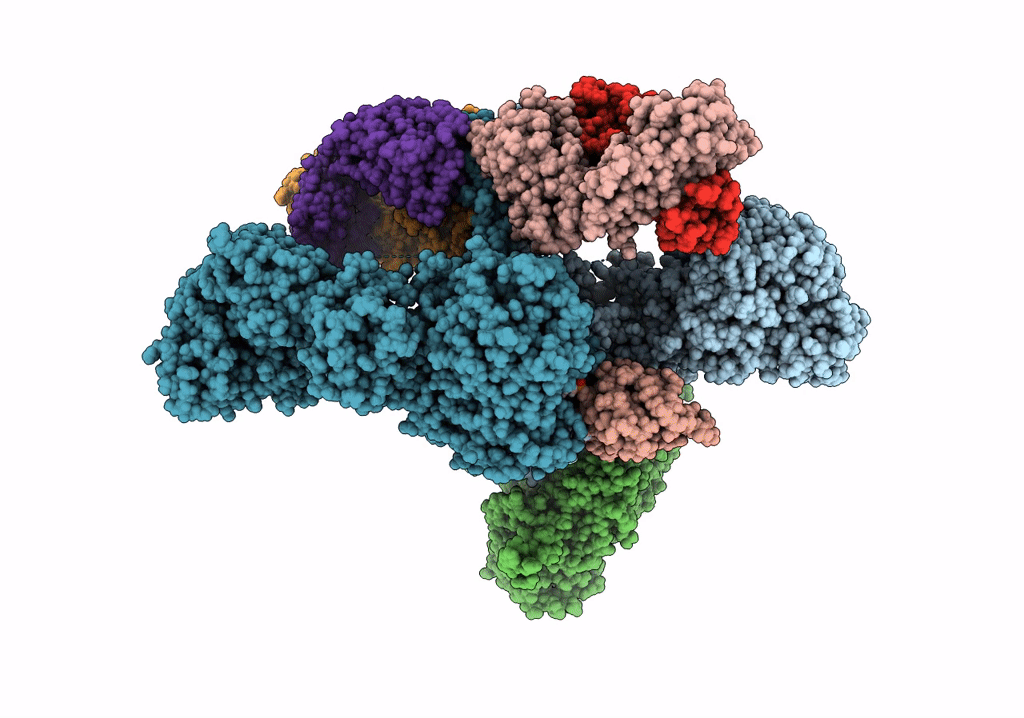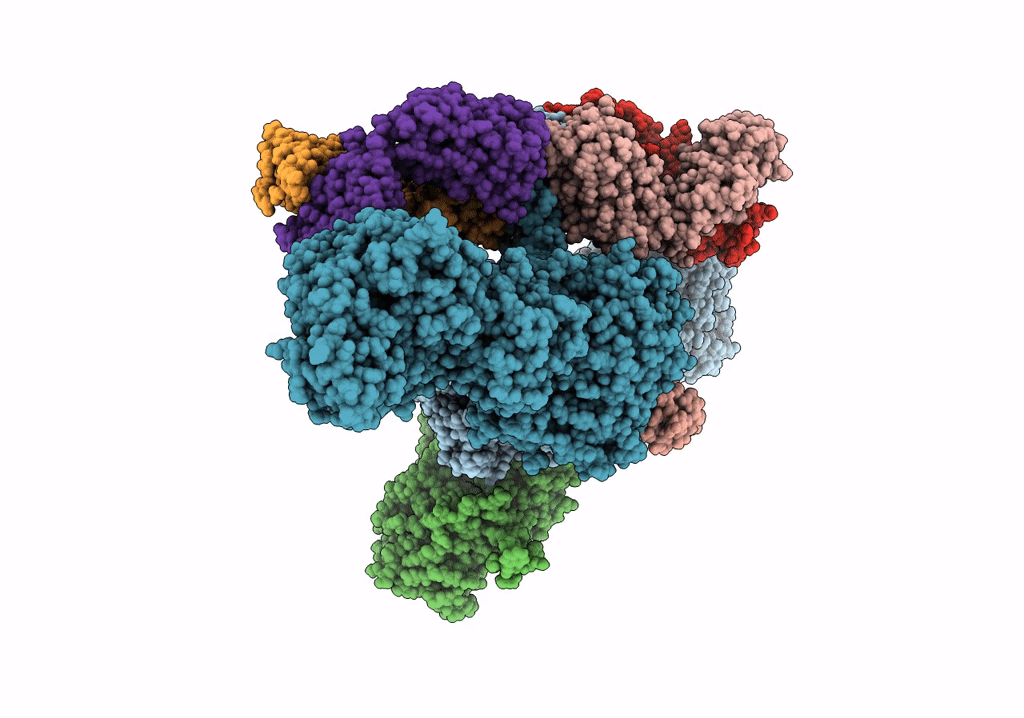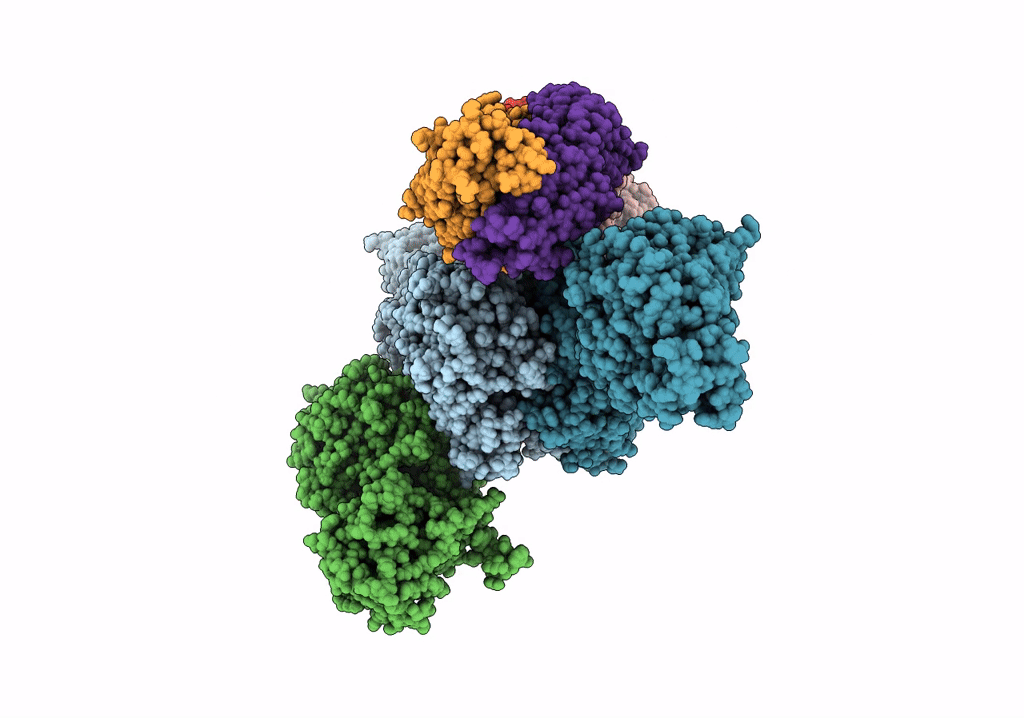
Title:
CryoEM structure of modular PKS holo-Lsd14 stalled at the condensation step and bound to antibody fragment 1B2, composite structure
Biological Source:
Source Organism(s):
Saccharopolyspora erythraea (Taxon ID: 1836)
Streptomyces lasalocidi (Taxon ID: 324833)
Homo sapiens (Taxon ID: 9606)
Streptomyces lasalocidi (Taxon ID: 324833)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE