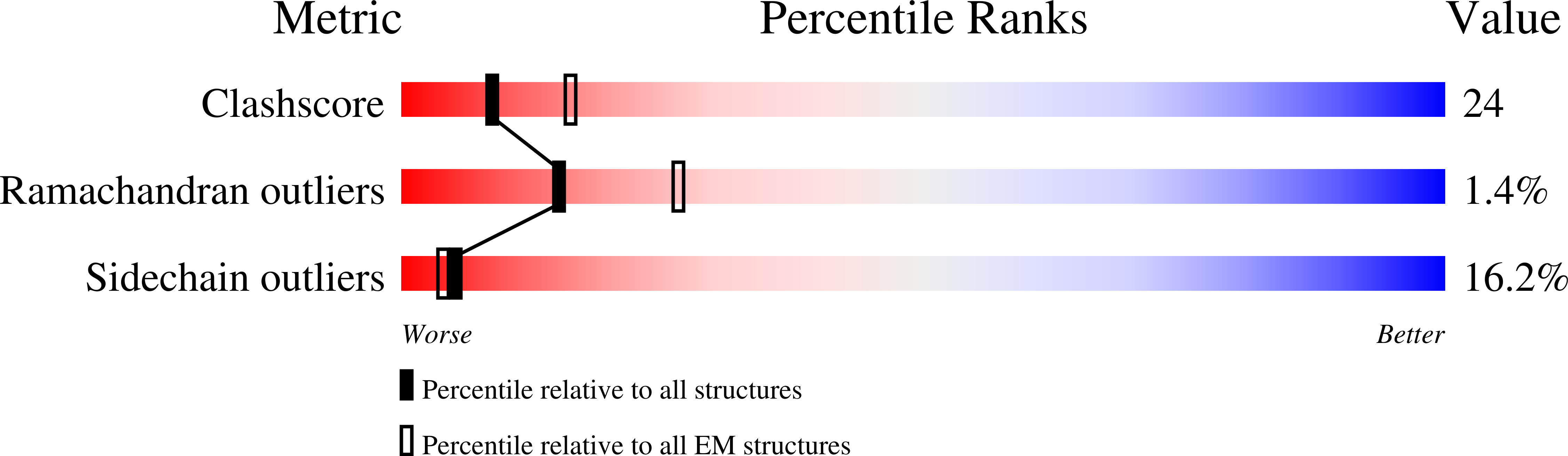
Deposition Date
2021-09-09
Release Date
2022-03-30
Last Version Date
2025-05-28
Entry Detail
PDB ID:
7S4L
Keywords:
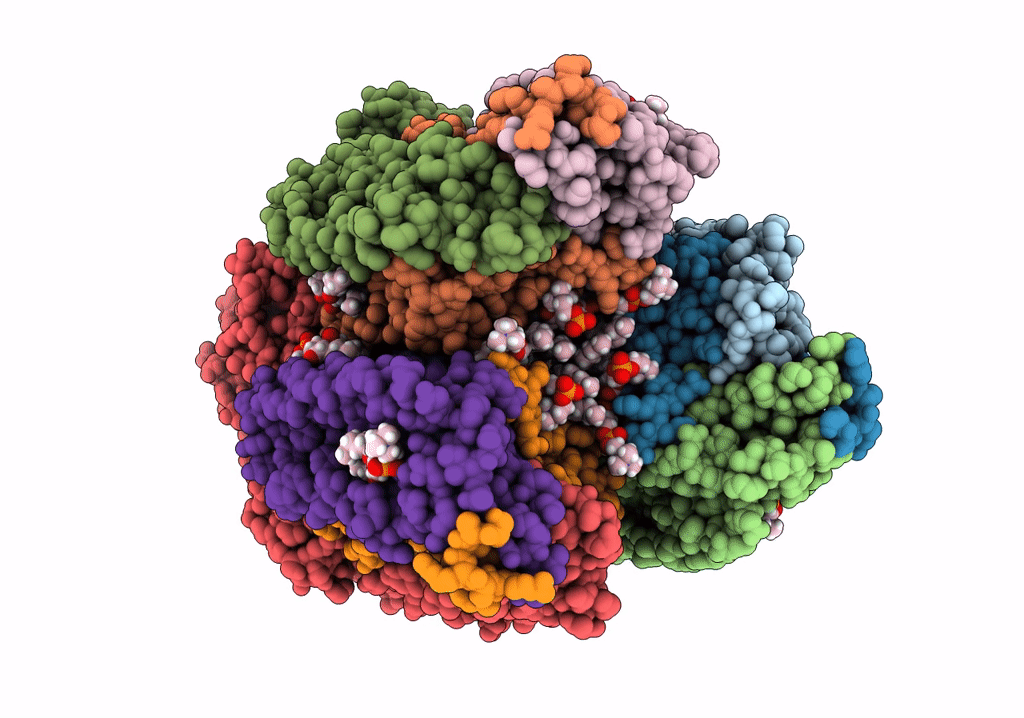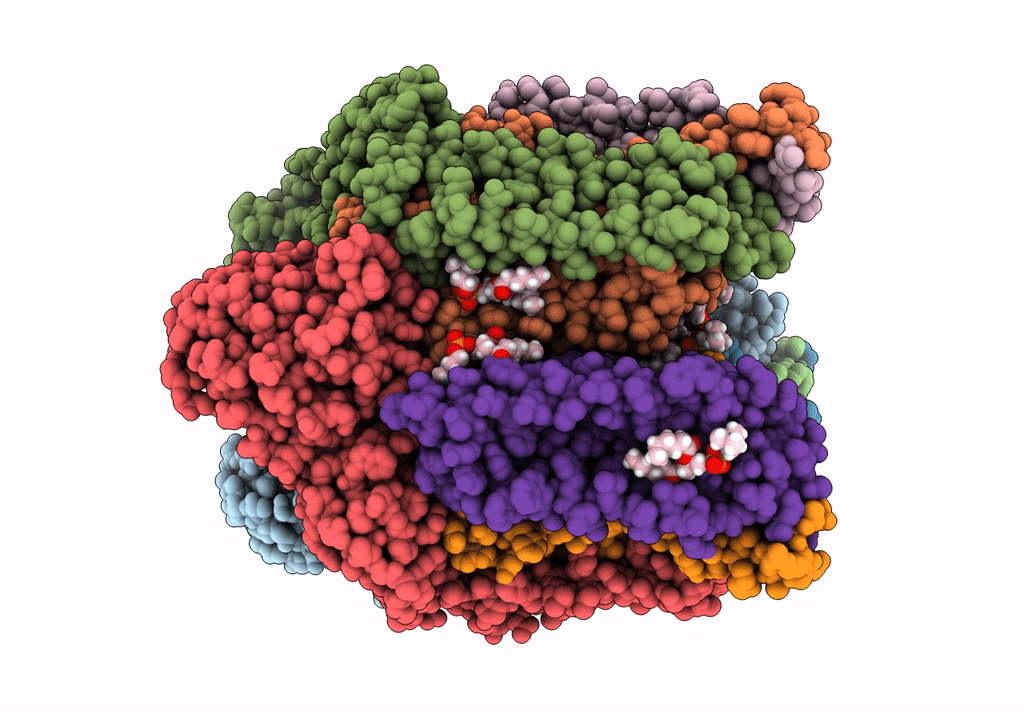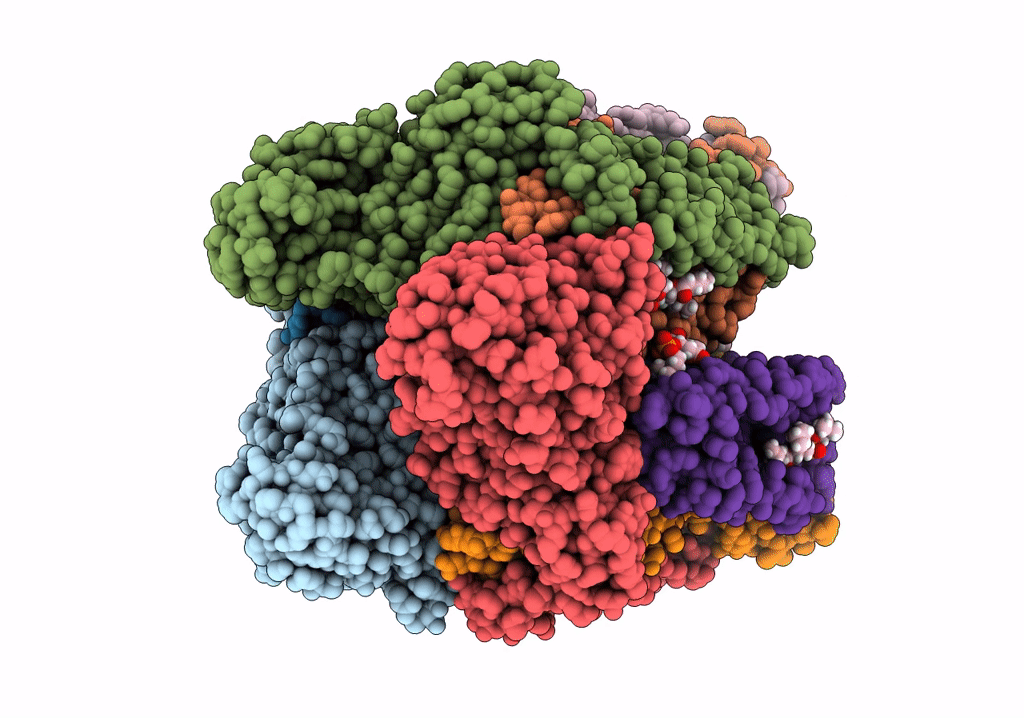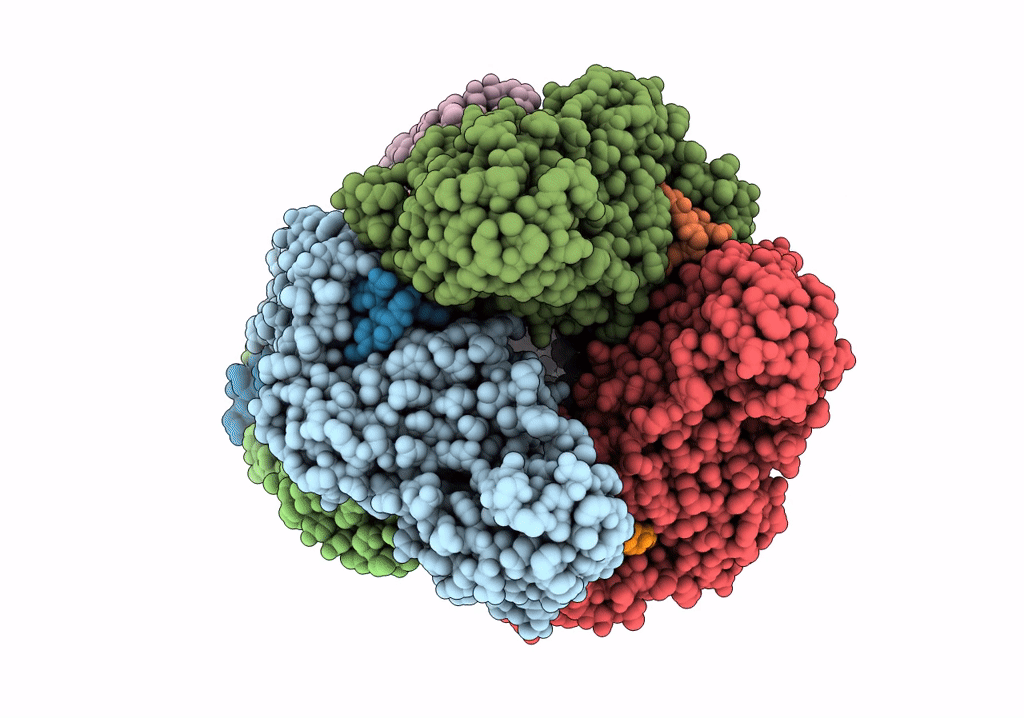
Title:
CryoEM structure of Methylotuvimicrobium alcaliphilum 20Z pMMO in a POPC nanodisc at 2.46 Angstrom resolution
Biological Source:
Source Organism(s):
Method Details:
Experimental Method:
Resolution:
2.46 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE