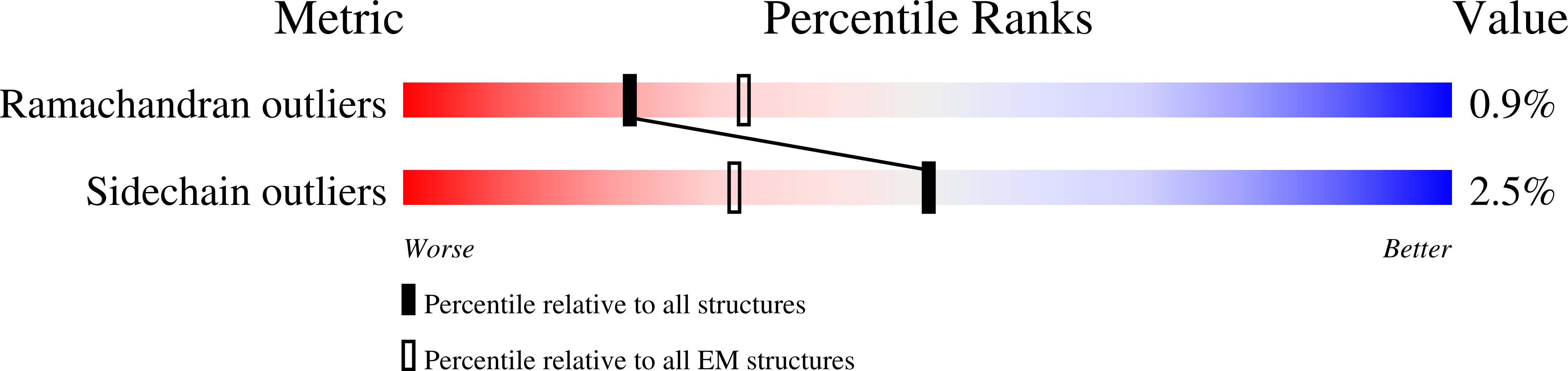
Deposition Date
2021-09-05
Release Date
2021-11-24
Last Version Date
2024-06-05
Entry Detail
PDB ID:
7S3D
Keywords:
Title:
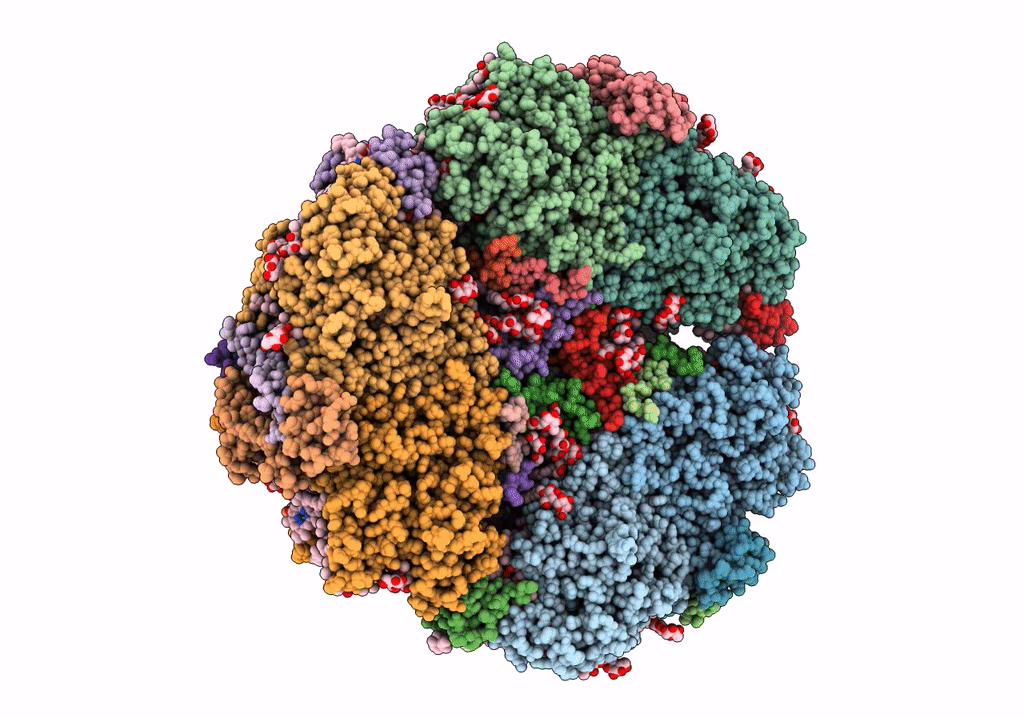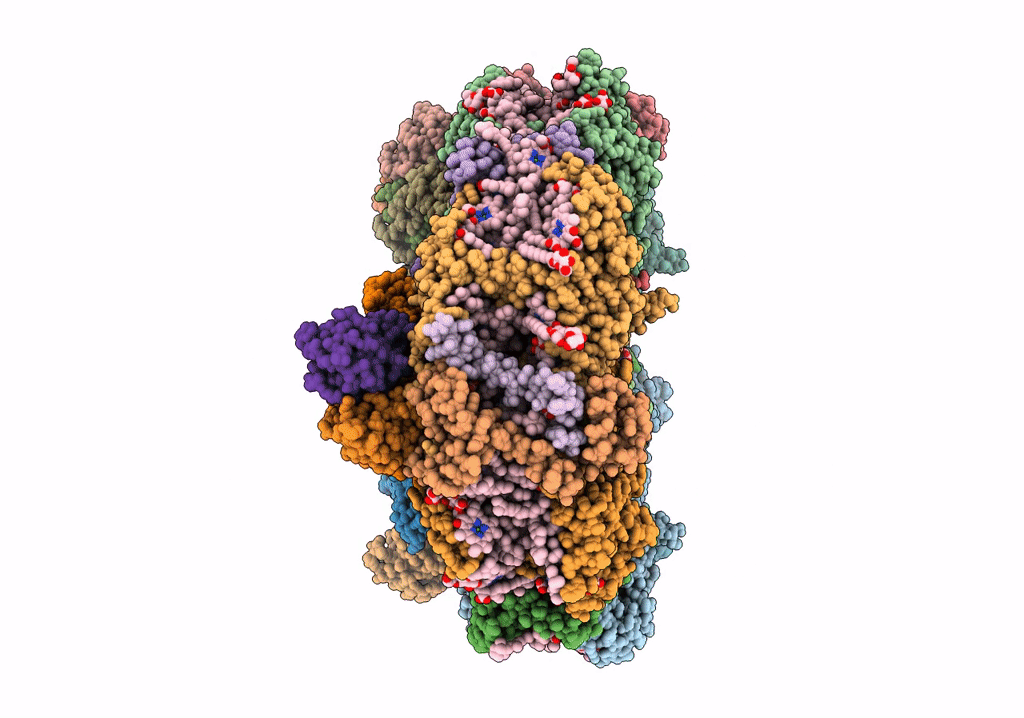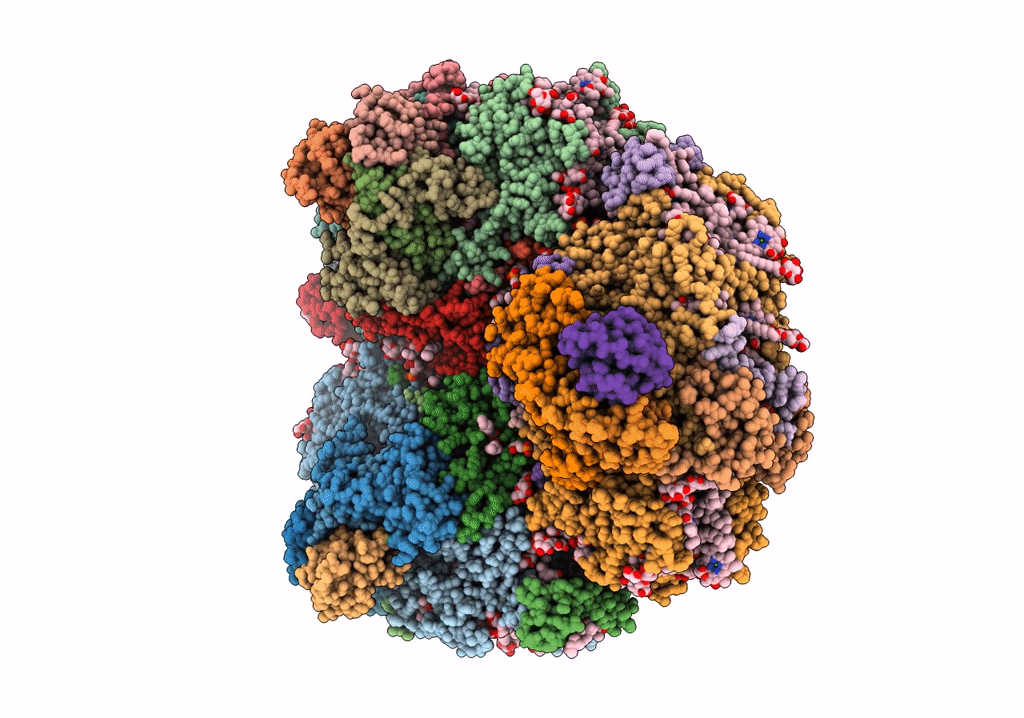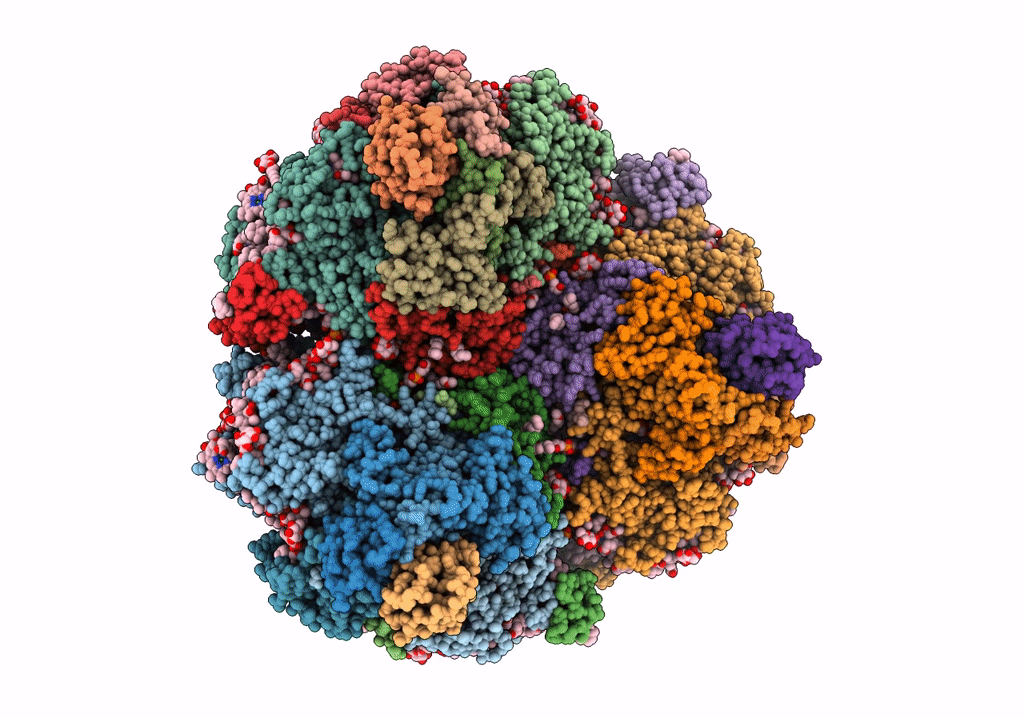
Structure of photosystem I with bound ferredoxin from Synechococcus sp. PCC 7335 acclimated to far-red light
Biological Source:
Source Organism(s):
Synechococcus sp. PCC 7335 (Taxon ID: 91464)
Method Details:
Experimental Method:
Resolution:
2.91 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE