Deposition Date
2022-02-07
Release Date
2022-12-14
Last Version Date
2024-01-31
Entry Detail
PDB ID:
7R3E
Keywords:
Title:
Fusion construct of PqsE and RhlR in complex with the synthetic antagonist mBTL
Biological Source:
Source Organism(s):
Pseudomonas aeruginosa PAO1 (Taxon ID: 208964)
Expression System(s):
Method Details:
Experimental Method:
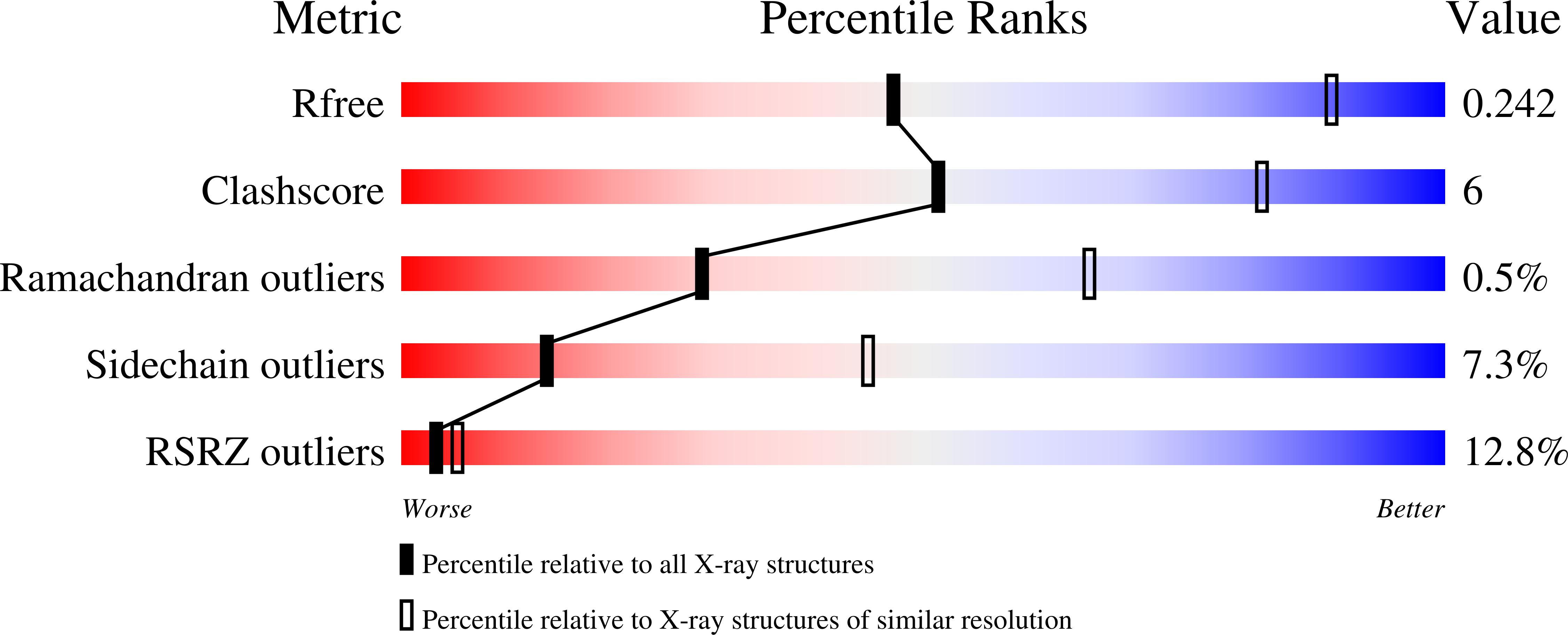
Resolution:
3.46 Å
R-Value Free:
0.24
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
I 4 2 2