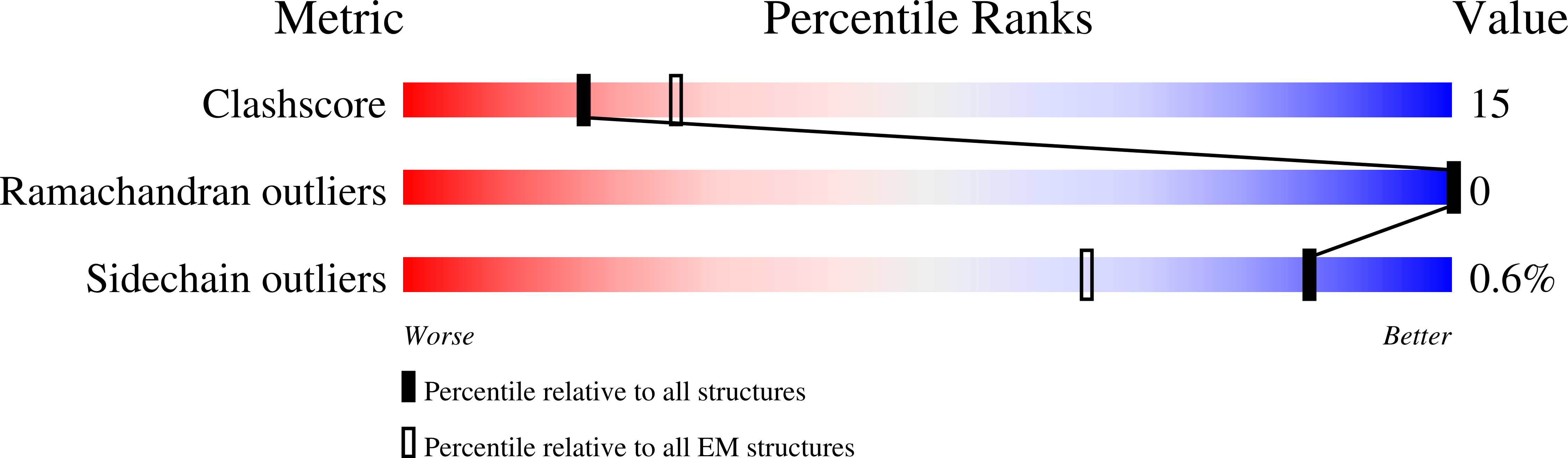Deposition Date
2022-01-18
Release Date
2022-09-21
Last Version Date
2023-10-04
Entry Detail
PDB ID:
7QUQ
Keywords:
Title:
BtubA(R284G,K286D,F287G):BtubB bacterial tubulin M-loop mutant forming a single protofilament (Prosthecobacter dejongeii)
Biological Source:
Source Organism:
Prosthecobacter dejongeii (Taxon ID: 48465)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.60 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE