Deposition Date
2021-12-15
Release Date
2022-03-16
Last Version Date
2022-03-16
Entry Detail
PDB ID:
7QIM
Keywords:
Title:
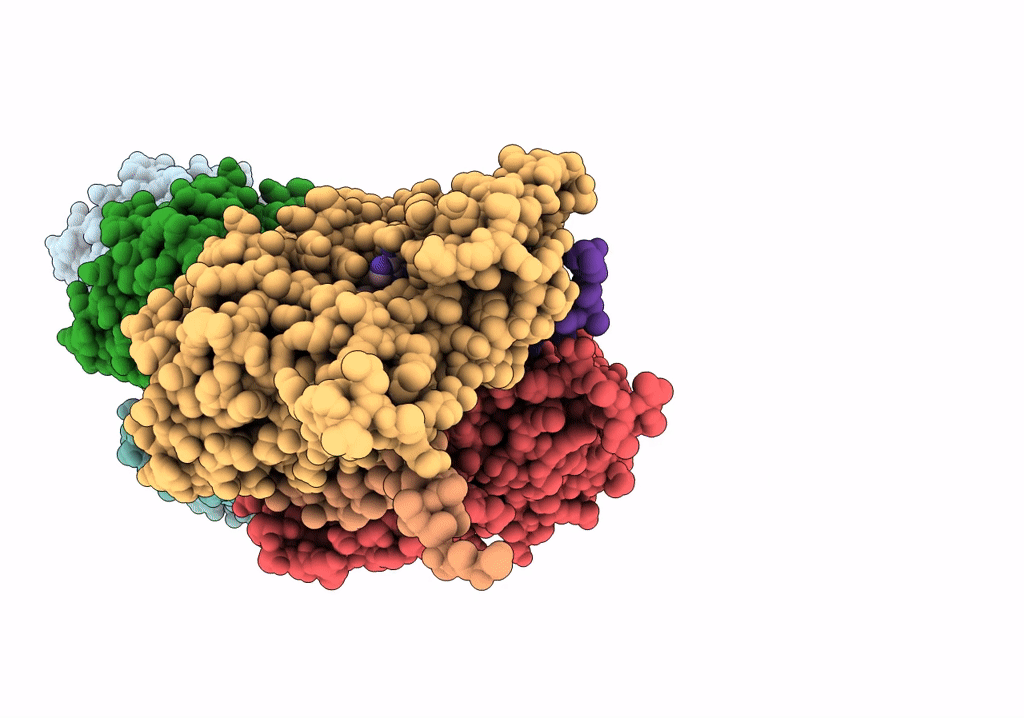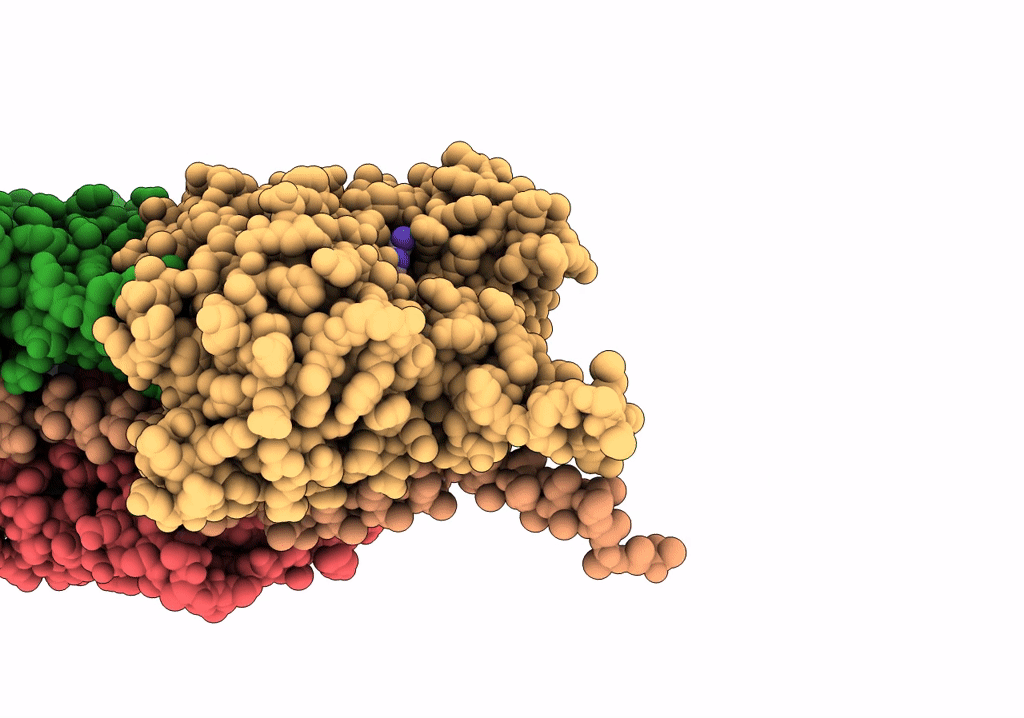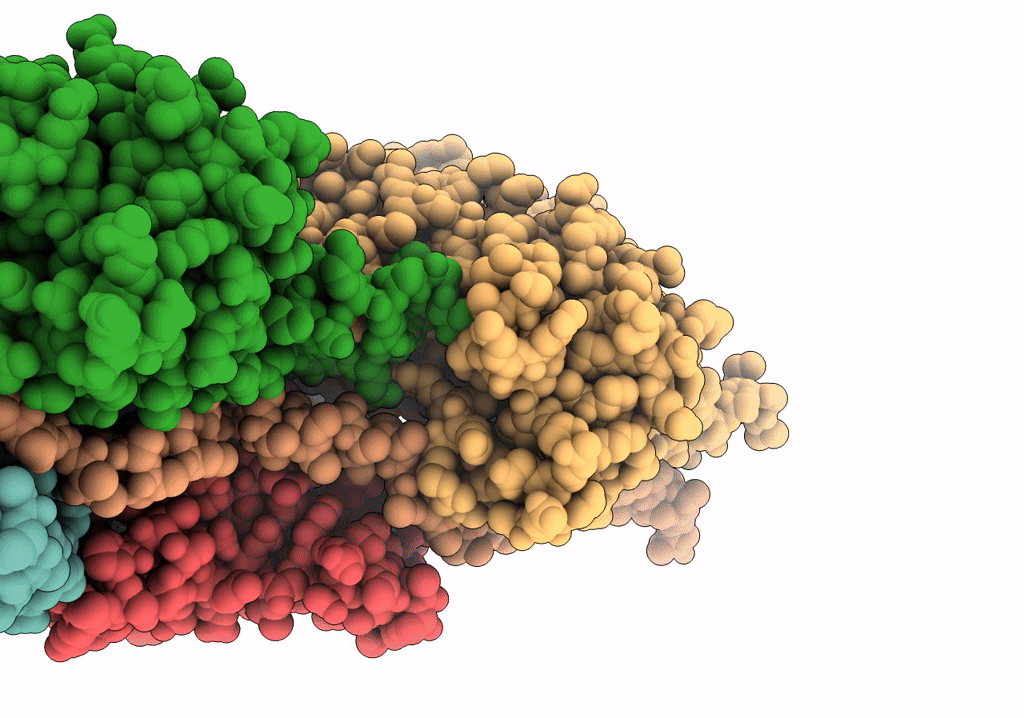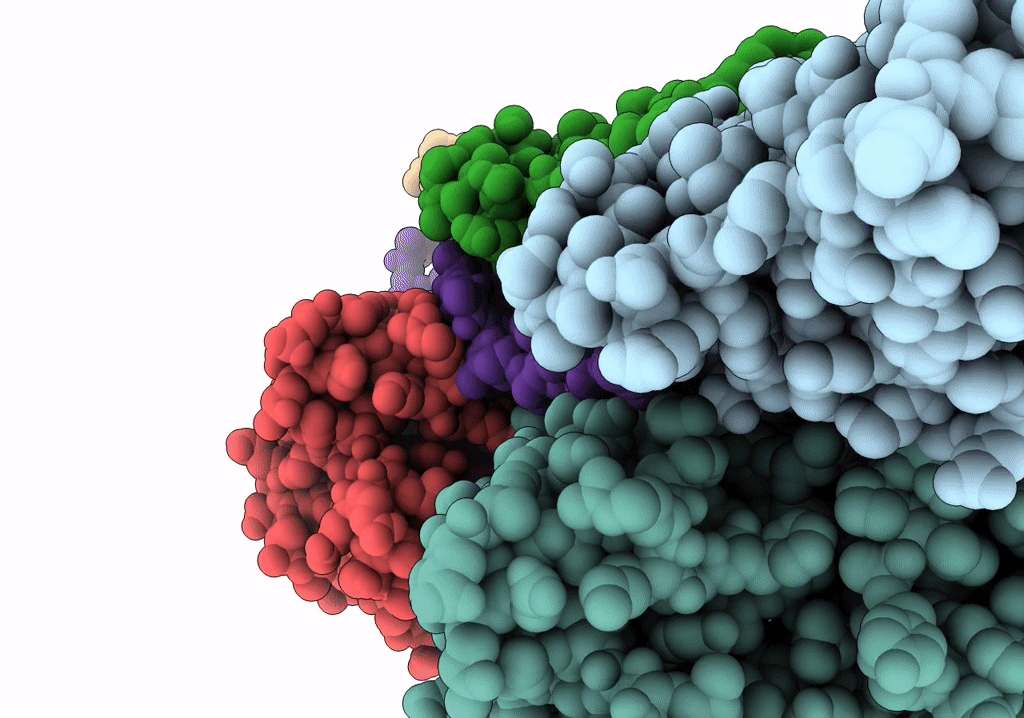
In situ structure of nebulin bound to actin filament in skeletal sarcomere
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Method Details:
Experimental Method:
Resolution:
4.50 Å
Aggregation State:
TISSUE
Reconstruction Method:
SUBTOMOGRAM AVERAGING


