Deposition Date
2021-12-10
Release Date
2022-12-21
Last Version Date
2024-11-20
Entry Detail
PDB ID:
7QH1
Keywords:
Title:
Discovery and development of a novel inhaled antivirulence therapy for the treatment of Pseudomonas aeruginosa infections in patients with chronic respiratory disease
Biological Source:
Source Organism:
Pseudomonas aeruginosa (Taxon ID: 287)
Method Details:
Experimental Method:
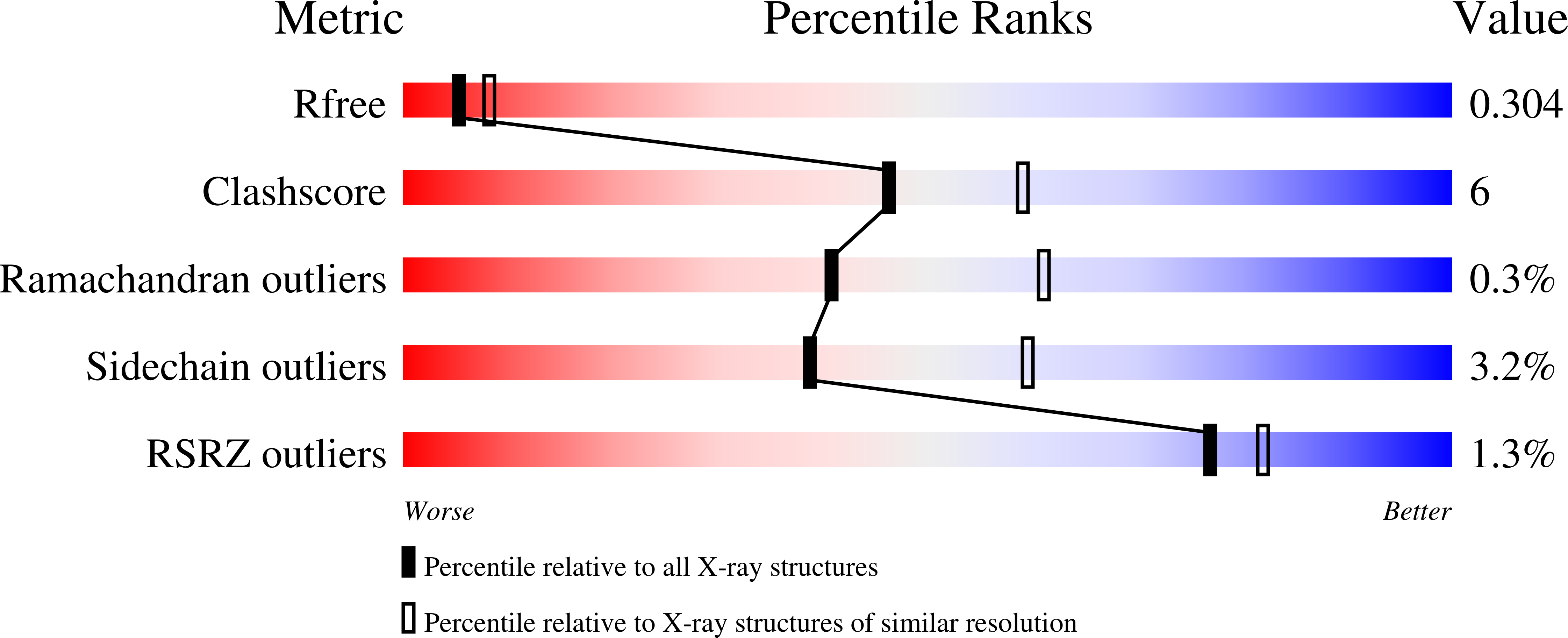
Resolution:
2.74 Å
R-Value Free:
0.30
R-Value Work:
0.25
R-Value Observed:
0.25
Space Group:
P 1 21 1