Deposition Date
2021-11-08
Release Date
2022-08-03
Last Version Date
2024-01-31
Entry Detail
PDB ID:
7Q6N
Keywords:
Title:
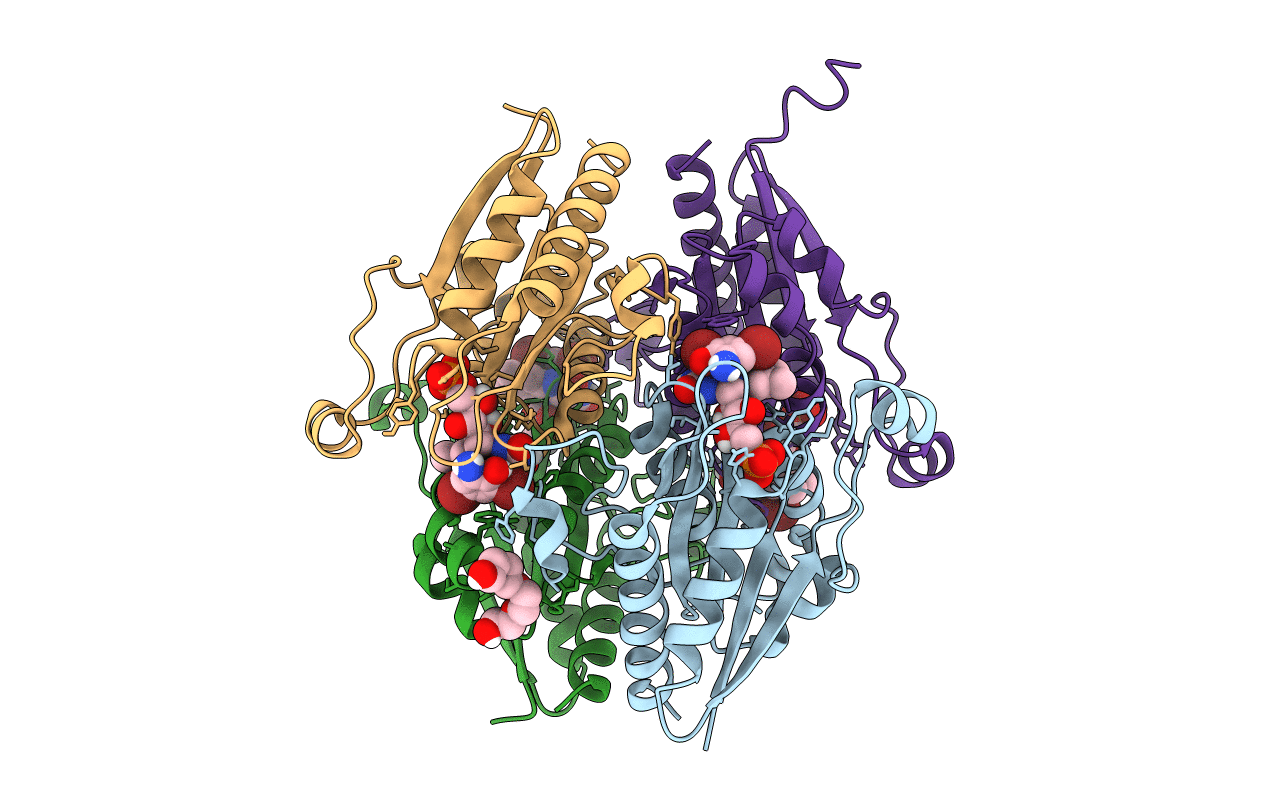
Structure of WrbA from Salmonella Typhimurium bound to ME0052
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
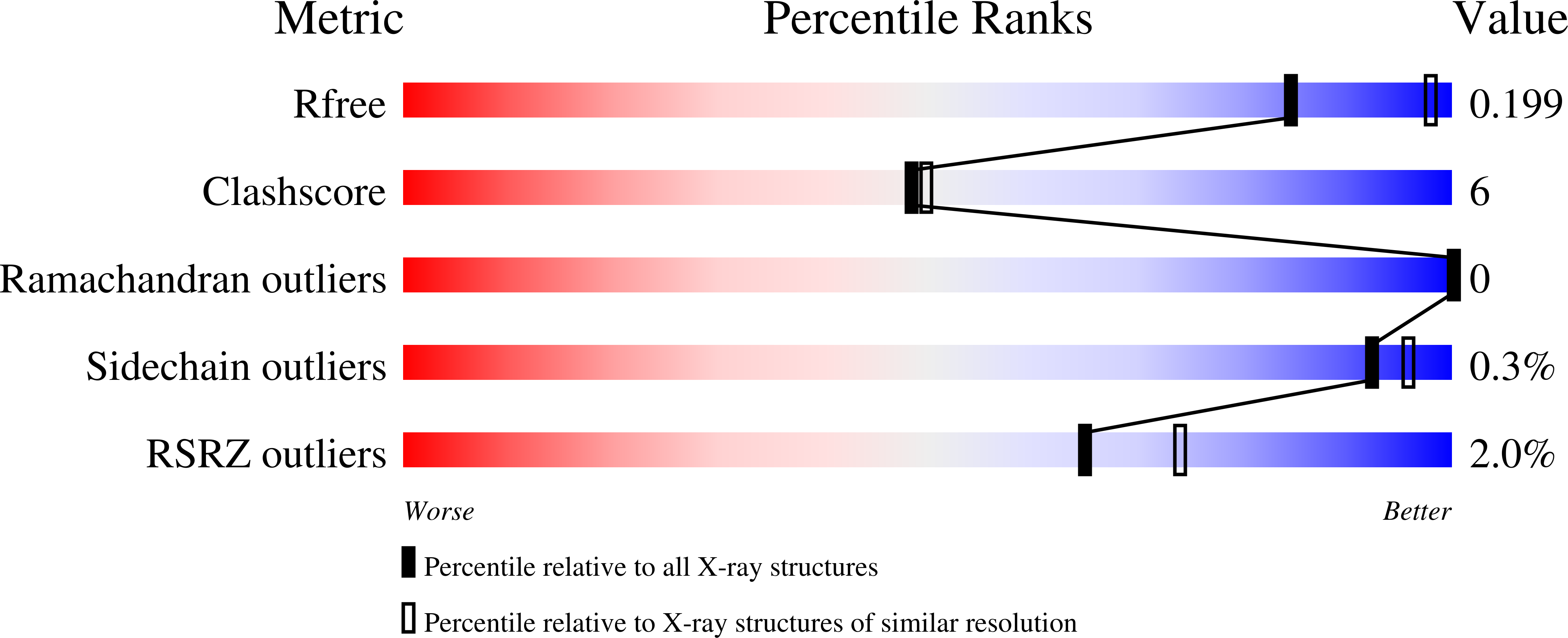
Resolution:
2.33 Å
R-Value Free:
0.19
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 21 21 21