Deposition Date
2021-10-05
Release Date
2022-11-16
Last Version Date
2025-09-10
Entry Detail
PDB ID:
7PVZ
Keywords:
Title:
Crystal structure of the intertwined dimer of the c-Src SH3 domain E93V-S94A-R95S-T96G mutant
Biological Source:
Source Organism(s):
Gallus gallus (Taxon ID: 9031)
Expression System(s):
Method Details:
Experimental Method:
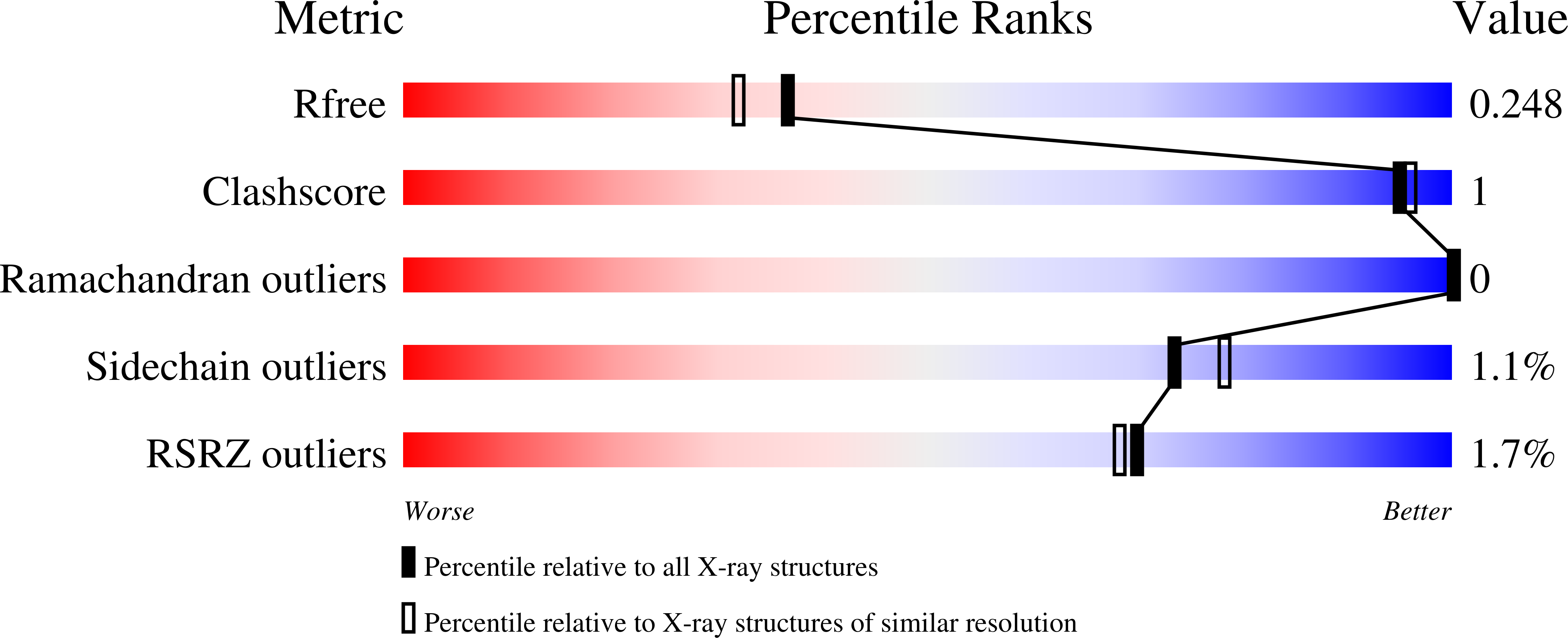
Resolution:
2.00 Å
R-Value Free:
0.25
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 64