Deposition Date
2021-09-08
Release Date
2022-09-21
Last Version Date
2024-02-28
Entry Detail
PDB ID:
7POC
Keywords:
Title:
An Irreversible, Promiscuous and Highly Thermostable Claisen-Condensation Biocatalyst Drives the Synthesis of Substituted Pyrroles
Biological Source:
Source Organism(s):
Thermus thermophilus (Taxon ID: 274)
Expression System(s):
Method Details:
Experimental Method:
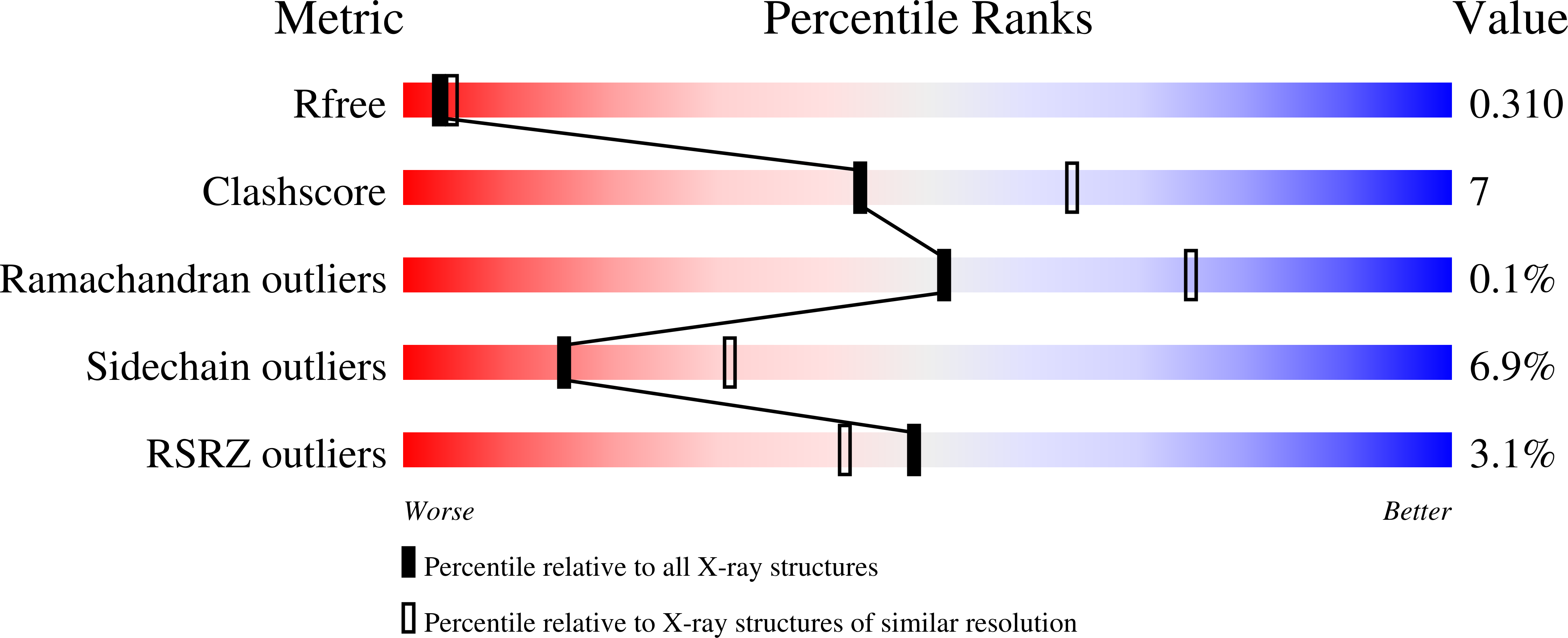
Resolution:
2.60 Å
R-Value Free:
0.31
R-Value Work:
0.24
Space Group:
P 21 21 21