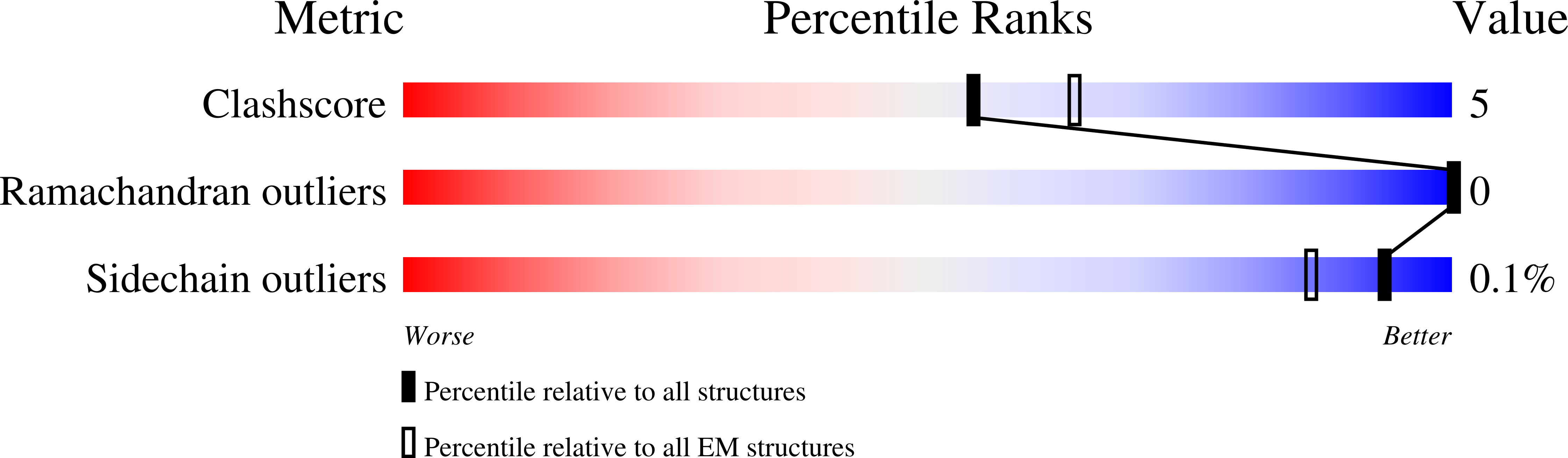
Deposition Date
2021-09-01
Release Date
2021-12-22
Last Version Date
2021-12-22
Entry Detail
PDB ID:
7PLX
Keywords:
Title:
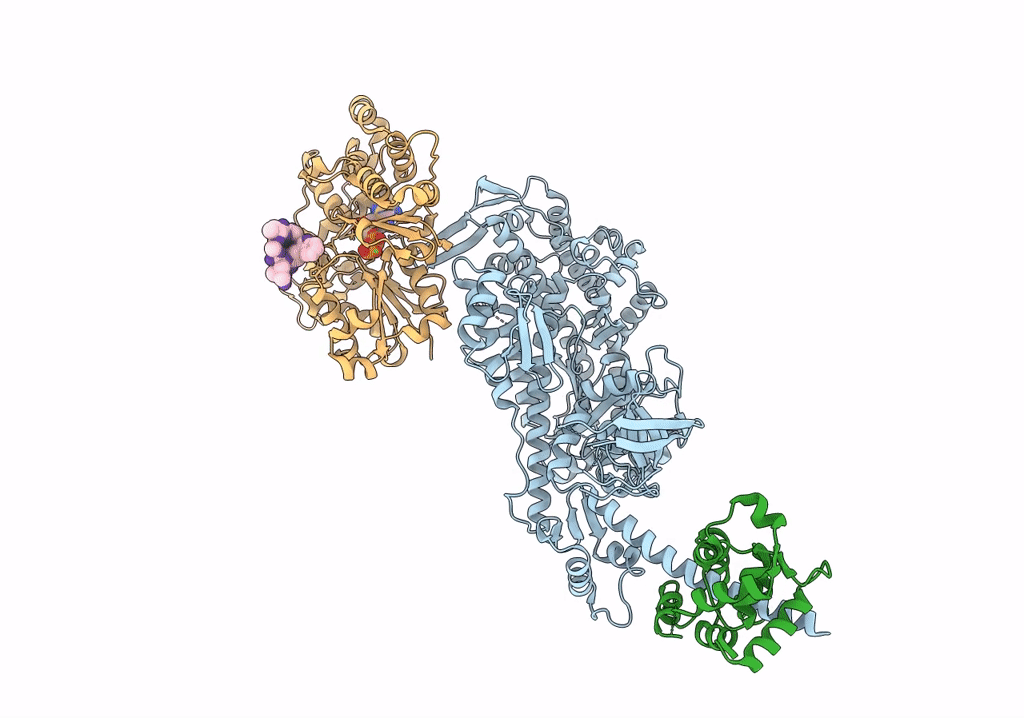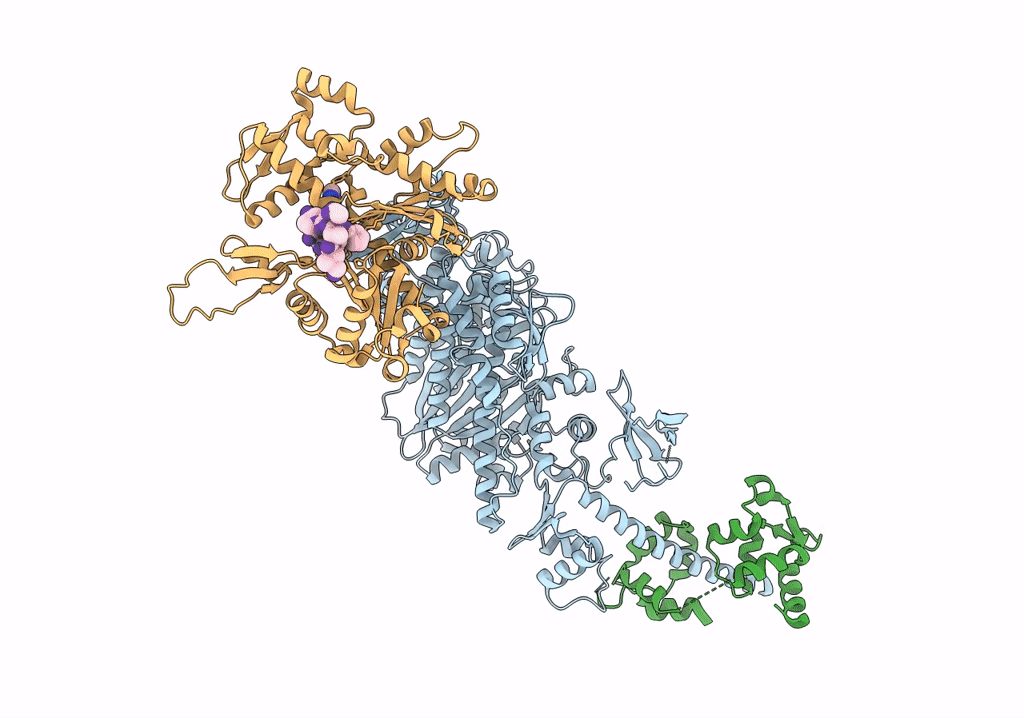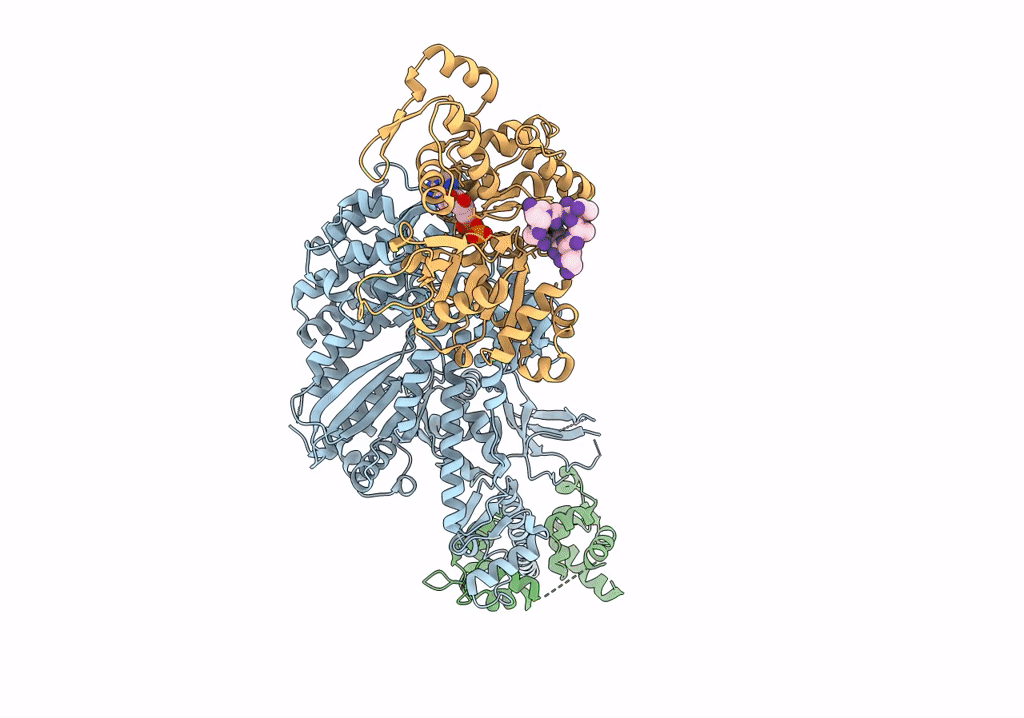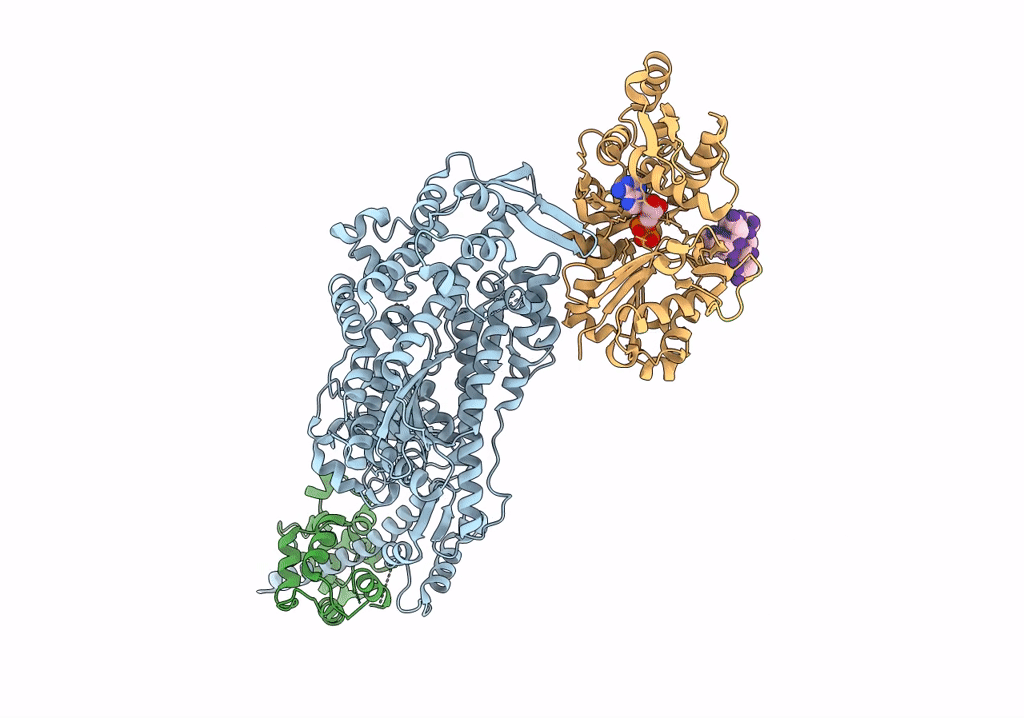
Cryo-EM structure of the actomyosin-V complex in the rigor state (central 1er, class 4)
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Gallus gallus (Taxon ID: 9031)
Amanita phalloides (Taxon ID: 67723)
Oryctolagus cuniculus (Taxon ID: 9986)
Gallus gallus (Taxon ID: 9031)
Amanita phalloides (Taxon ID: 67723)
Oryctolagus cuniculus (Taxon ID: 9986)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.60 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL