Deposition Date
2021-08-14
Release Date
2022-08-24
Last Version Date
2025-12-10
Entry Detail
PDB ID:
7PGJ
Keywords:
Title:
Chimeric carminomycin-4-O-methyltransferase (DnrK) with regions from 10-decarboxylate TamK and 10-hydroxylase RdmB, together with a single point mutation F297G
Biological Source:
Source Organism:
Streptomyces peucetius (Taxon ID: 1950)
Streptomyces tsukubensis (strain DSM 42081 / NBRC 108919 / NRRL 18488 / 9993) (Taxon ID: 1114943)
Streptomyces purpurascens (Taxon ID: 1924)
Streptomyces tsukubensis (strain DSM 42081 / NBRC 108919 / NRRL 18488 / 9993) (Taxon ID: 1114943)
Streptomyces purpurascens (Taxon ID: 1924)
Host Organism:
Method Details:
Experimental Method:
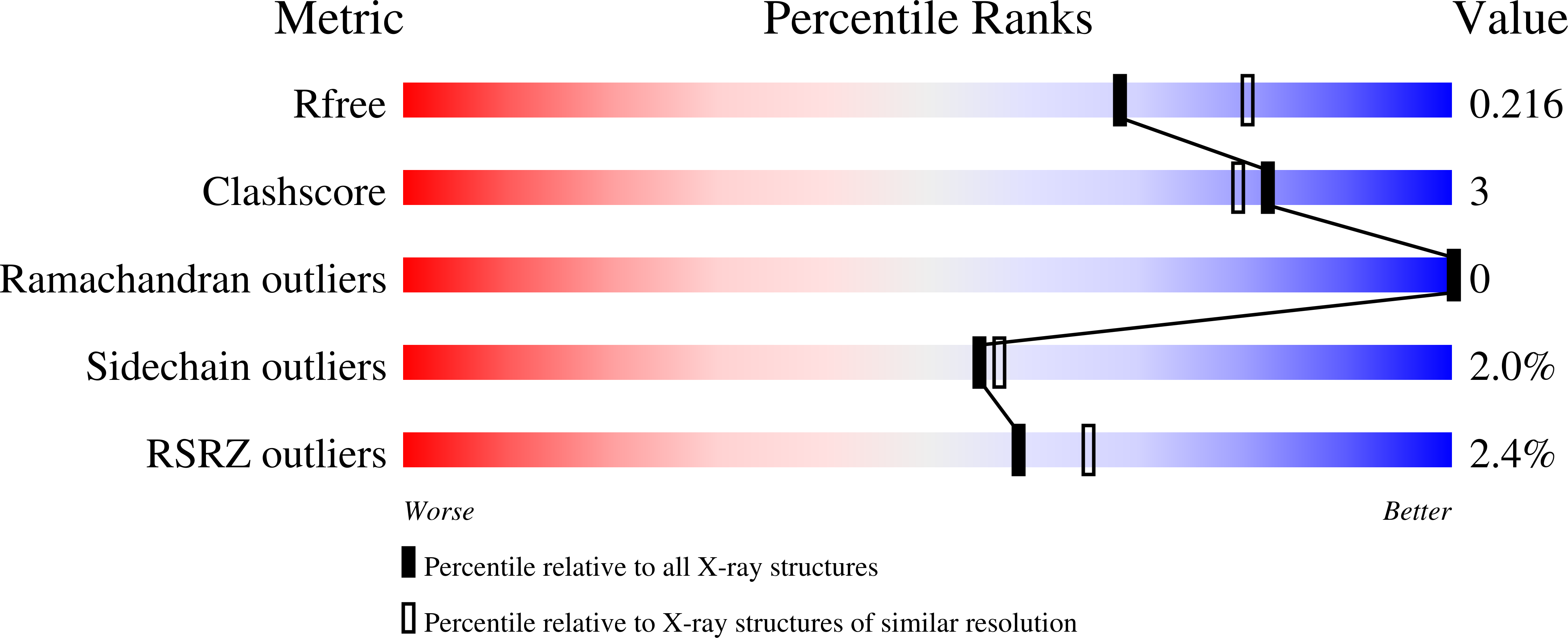
Resolution:
2.13 Å
R-Value Free:
0.21
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
C 2 2 21