Deposition Date
2021-08-12
Release Date
2022-01-26
Last Version Date
2024-11-13
Entry Detail
PDB ID:
7PFS
Keywords:
Title:
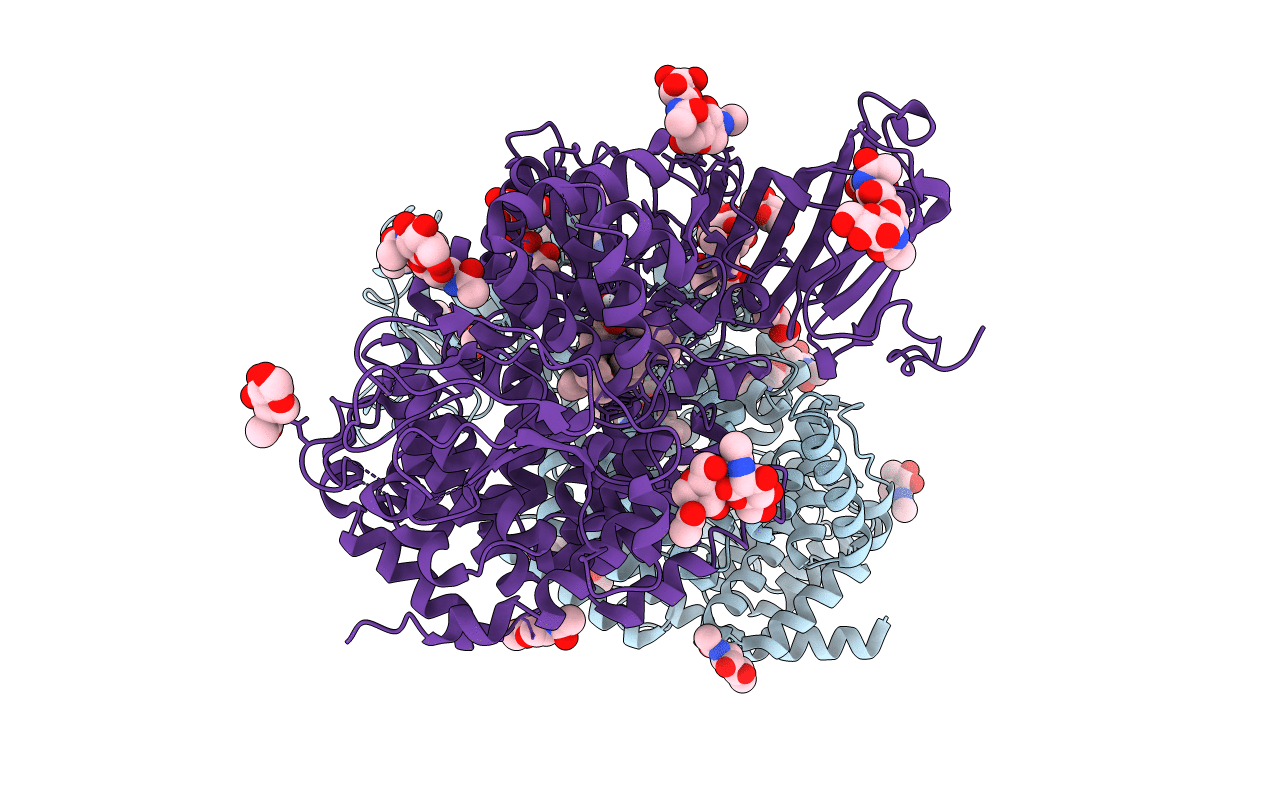
Crystal structure of ERAP2 aminopeptidase in complex with phosphinic pseudotripeptide ((1R)-1-Amino-3-phenylpropyl){2-([1,1:3,1-terphenyl]-5-ylmethyl)-3-[((2S)-1-amino-1-oxo-3-phenylpropan-2-yl)-amino]-3-oxopropyl}phosphinic acid
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
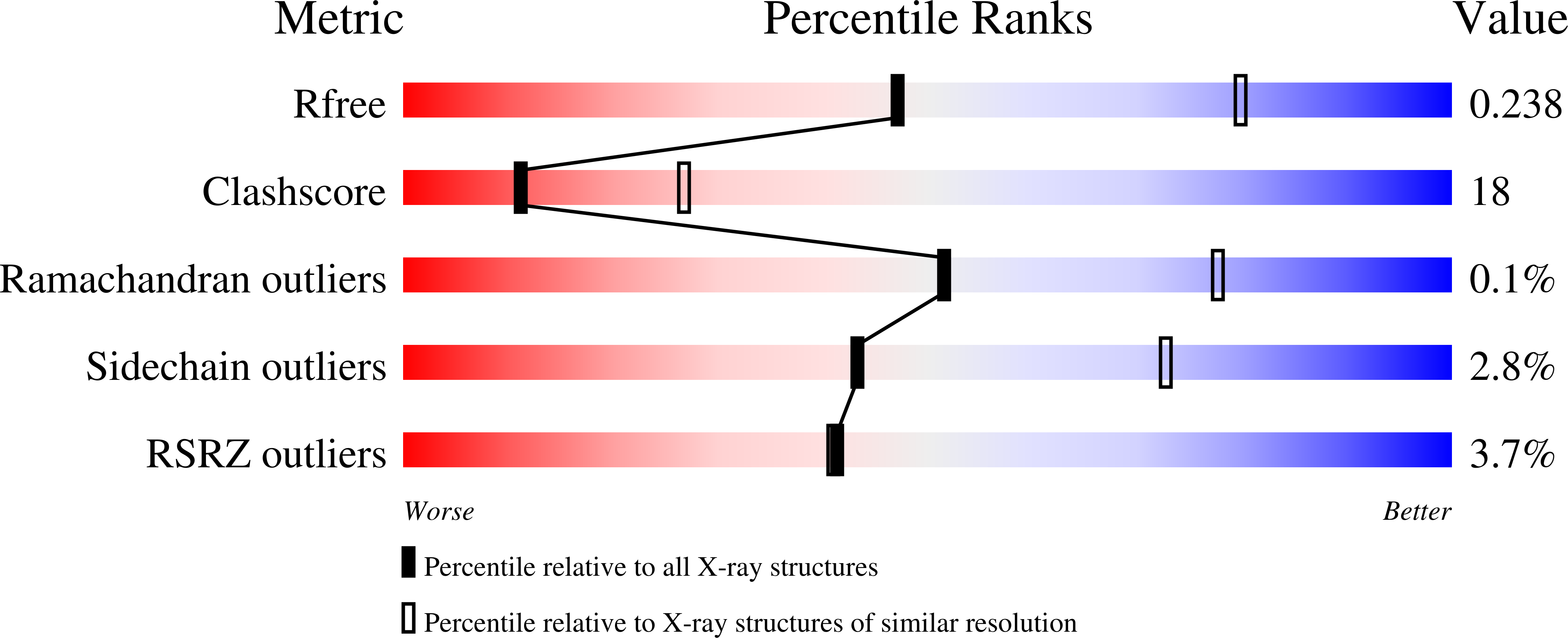
Resolution:
2.70 Å
R-Value Free:
0.23
R-Value Work:
0.18
R-Value Observed:
0.19
Space Group:
P 1 21 1