Deposition Date
2021-07-07
Release Date
2022-05-11
Last Version Date
2024-07-17
Entry Detail
PDB ID:
7P3F
Keywords:
Title:
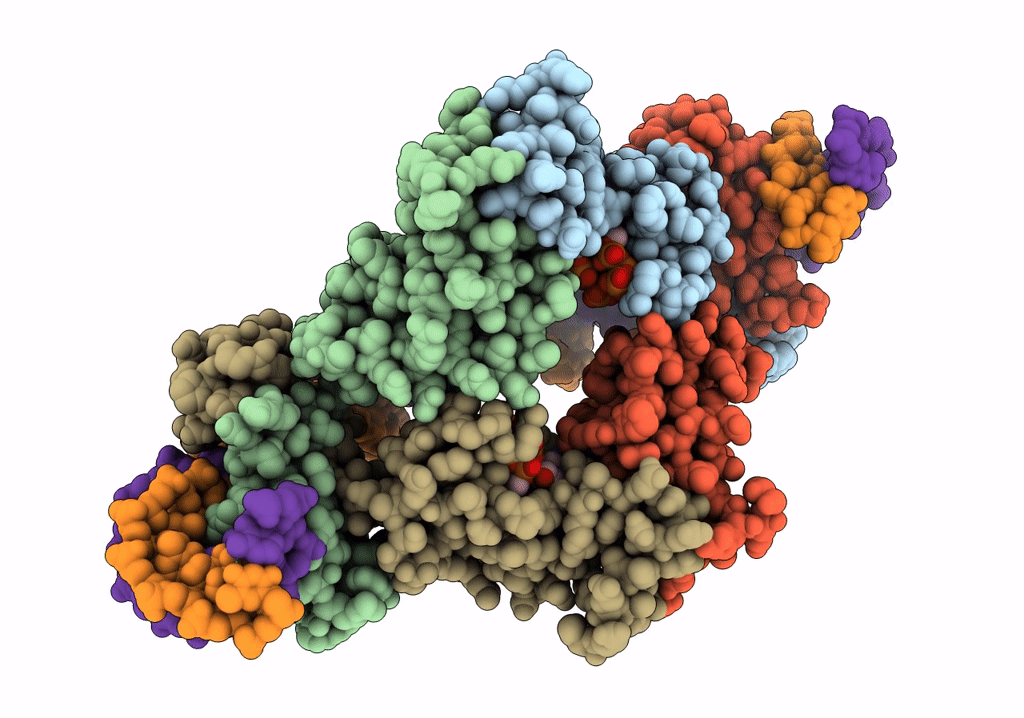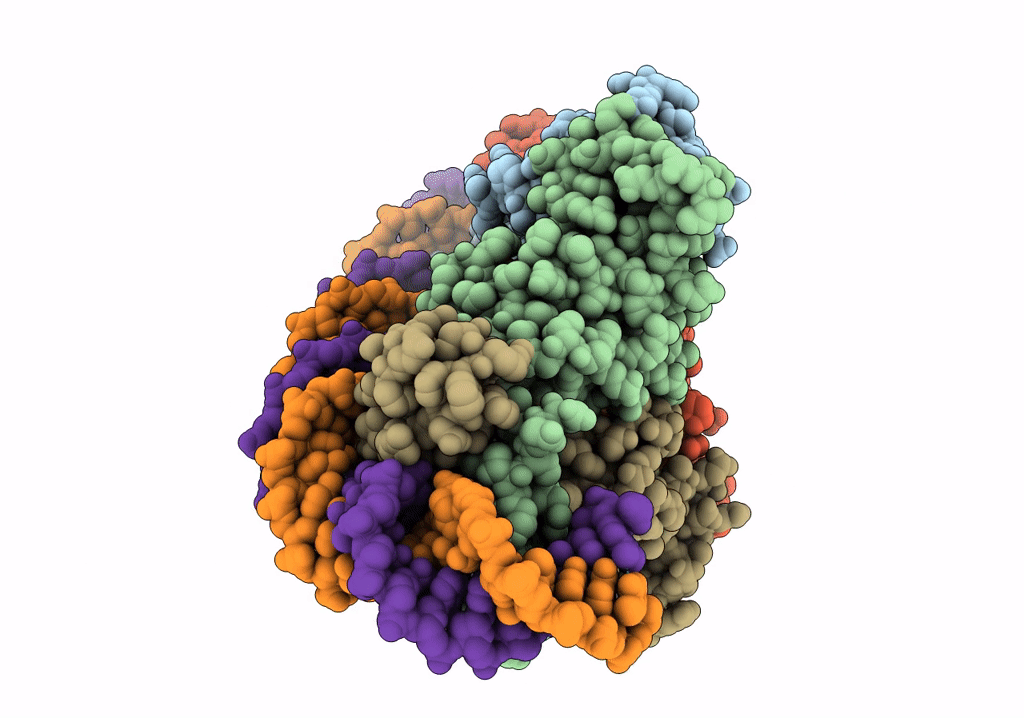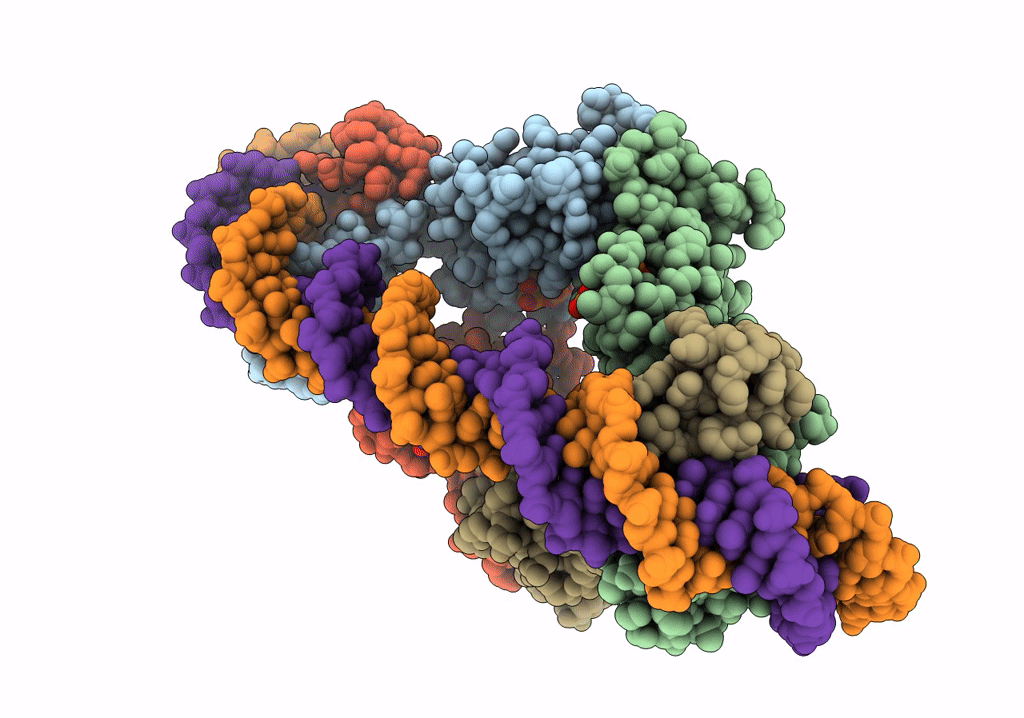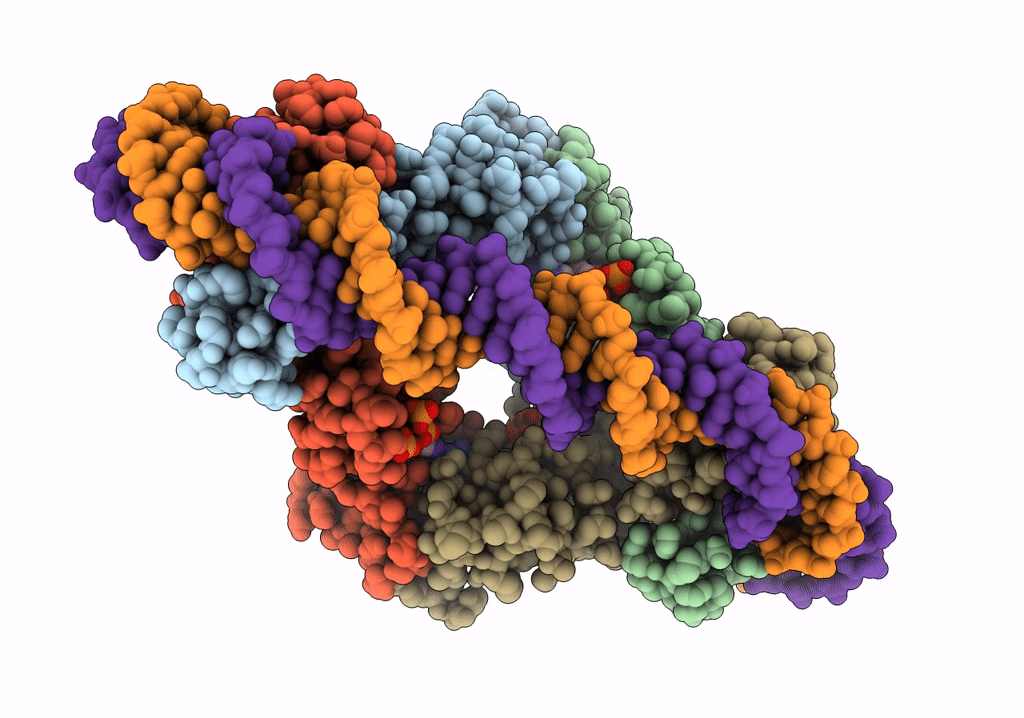
Streptomyces coelicolor dATP/ATP-loaded NrdR in complex with its cognate DNA
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.31 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


