Deposition Date
2021-06-10
Release Date
2021-12-01
Last Version Date
2024-07-17
Entry Detail
PDB ID:
7OTT
Keywords:
Title:
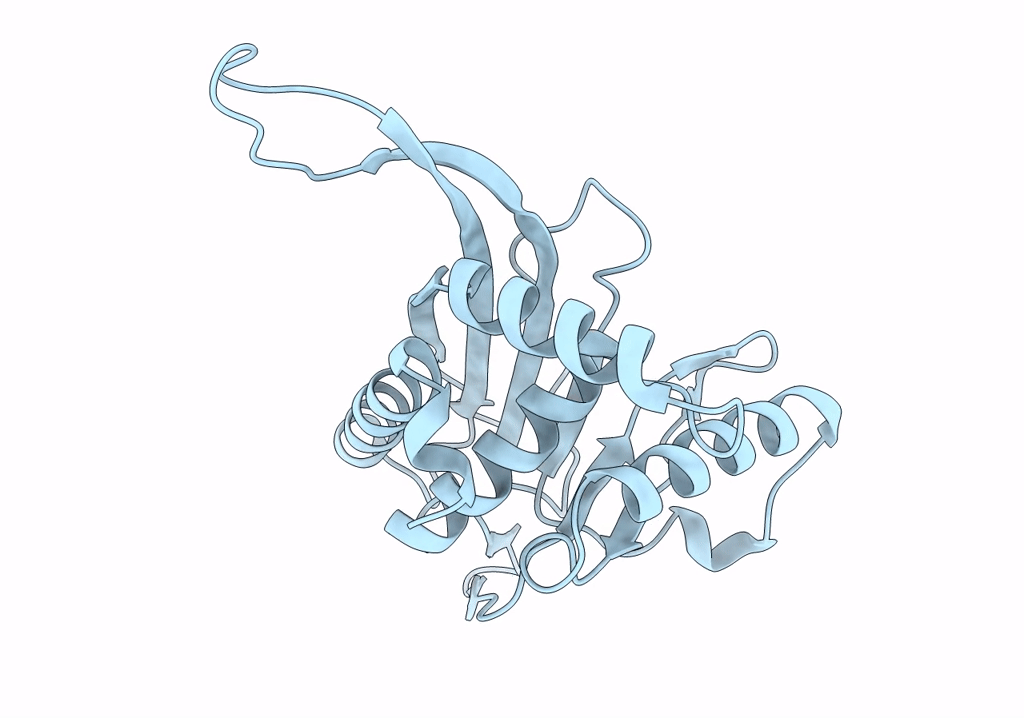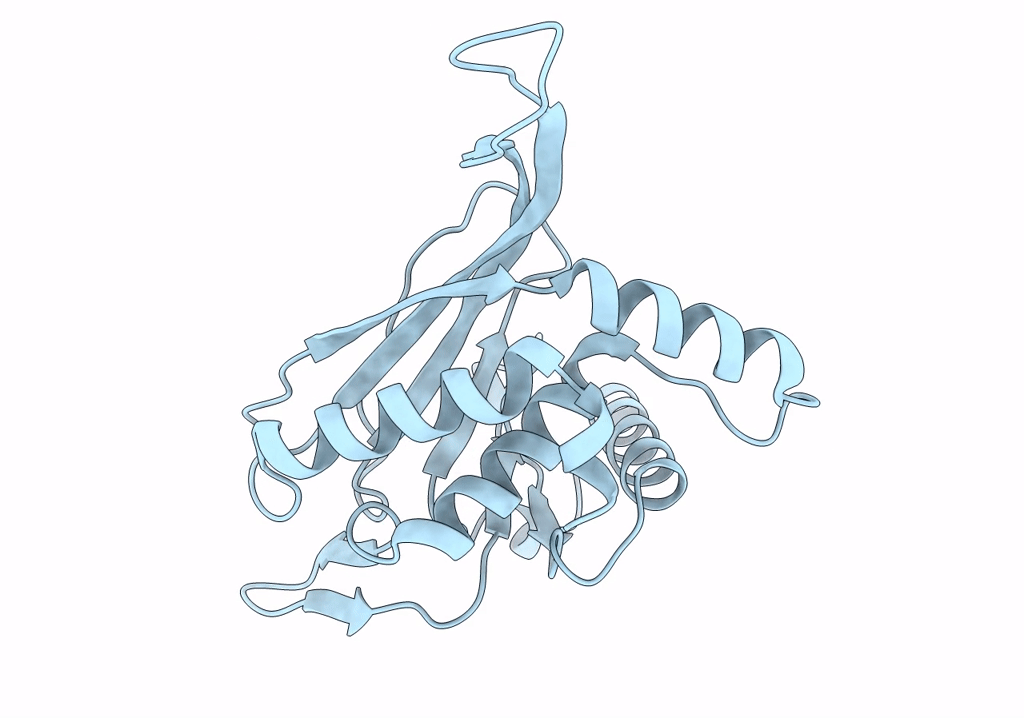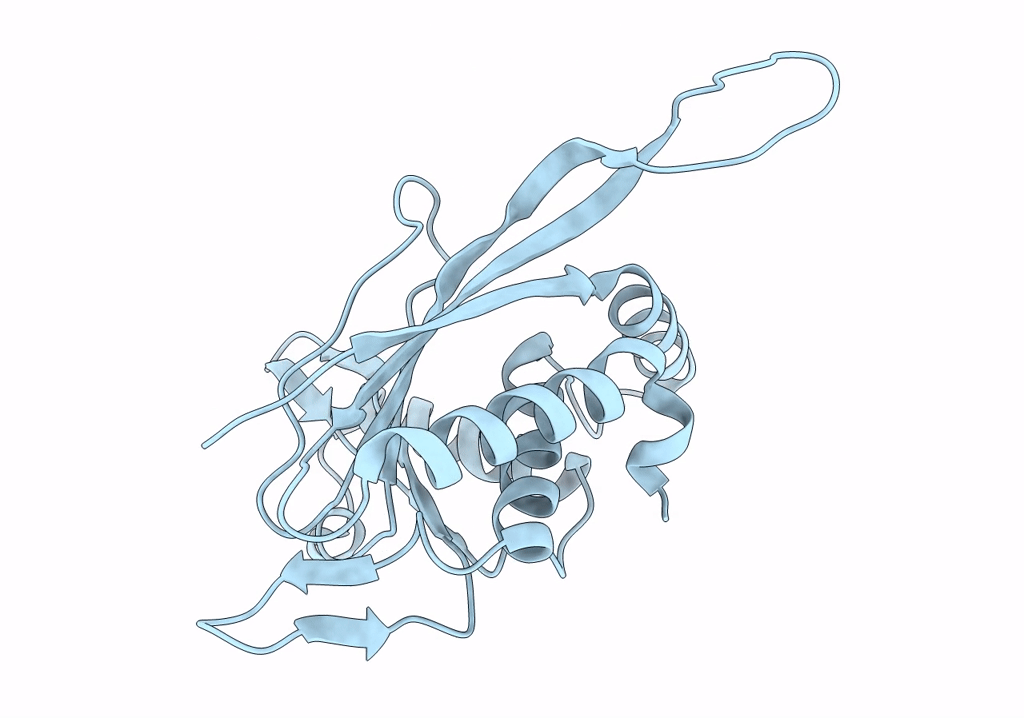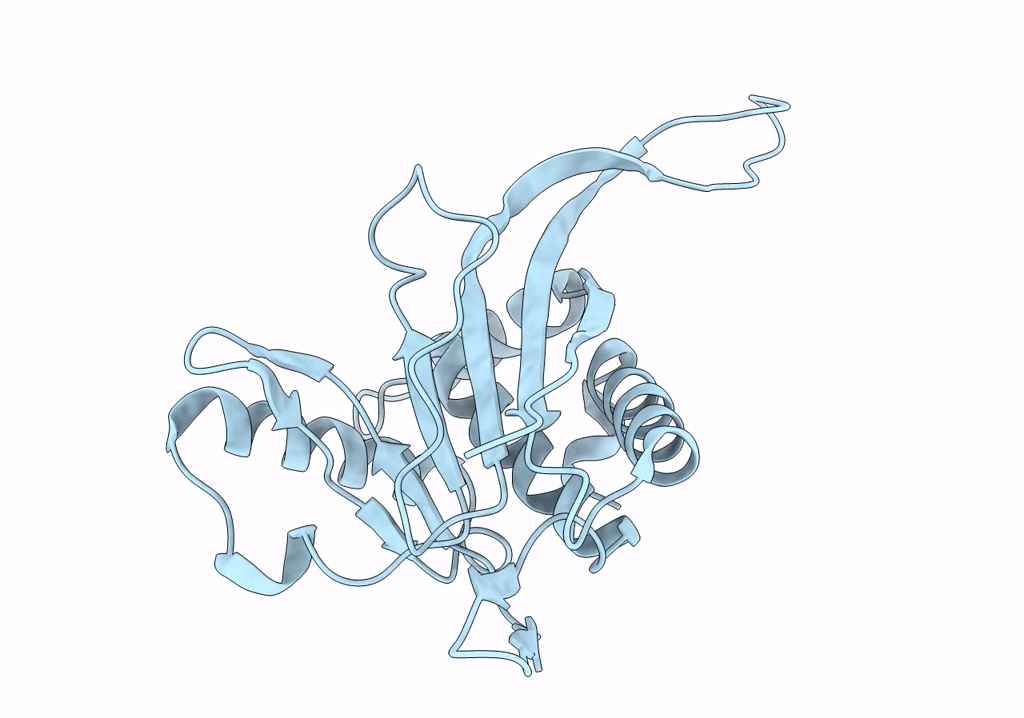
Metabolon-embedded pyruvate dehydrogenase complex E2 core at near-atomic resolution
Biological Source:
Source Organism(s):
Method Details:
Experimental Method:
Resolution:
3.84 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


