Deposition Date
2021-05-06
Release Date
2021-07-07
Last Version Date
2024-07-10
Entry Detail
PDB ID:
7OGM
Keywords:
Title:
A cooperative PNPase-Hfq-RNA carrier complex facilitates bacterial riboregulation. PNPase-3'ETS(leuZ)-Hfq
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
Escherichia coli (strain K12) (Taxon ID: 83333)
Escherichia coli (strain K12) (Taxon ID: 83333)
Expression System(s):
Method Details:
Experimental Method:
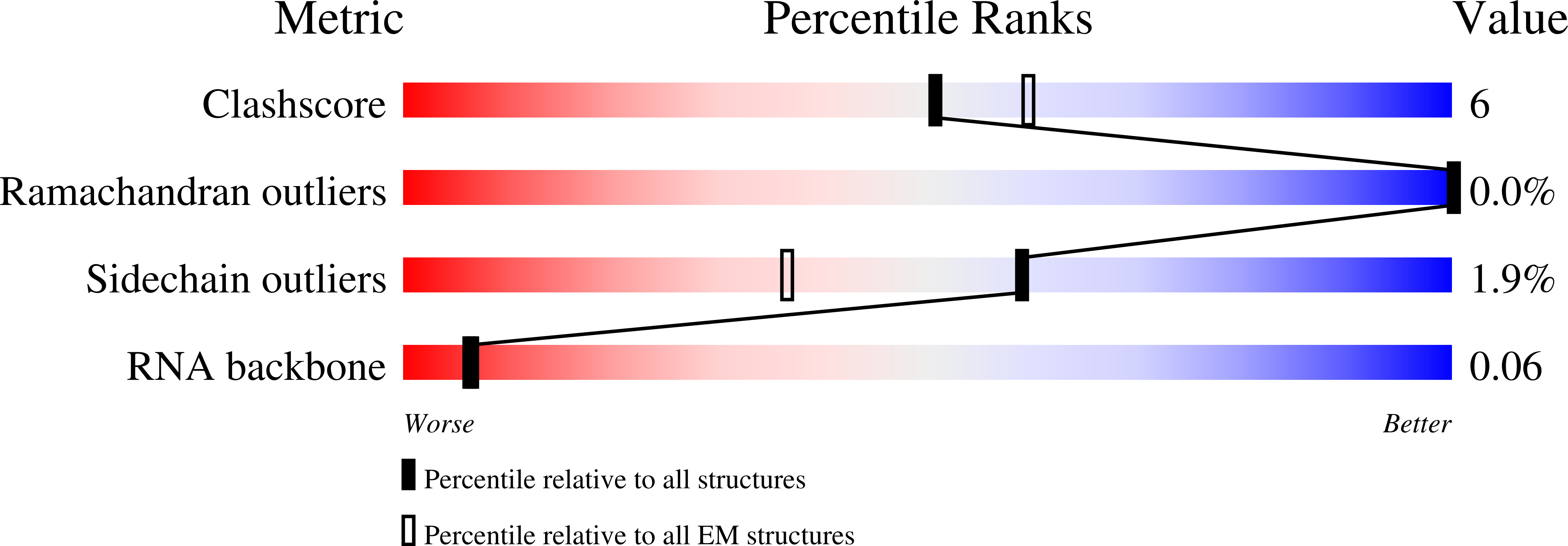
Resolution:
3.70 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE