Deposition Date
2021-04-19
Release Date
2021-11-24
Last Version Date
2024-07-10
Entry Detail
PDB ID:
7OA6
Keywords:
Title:
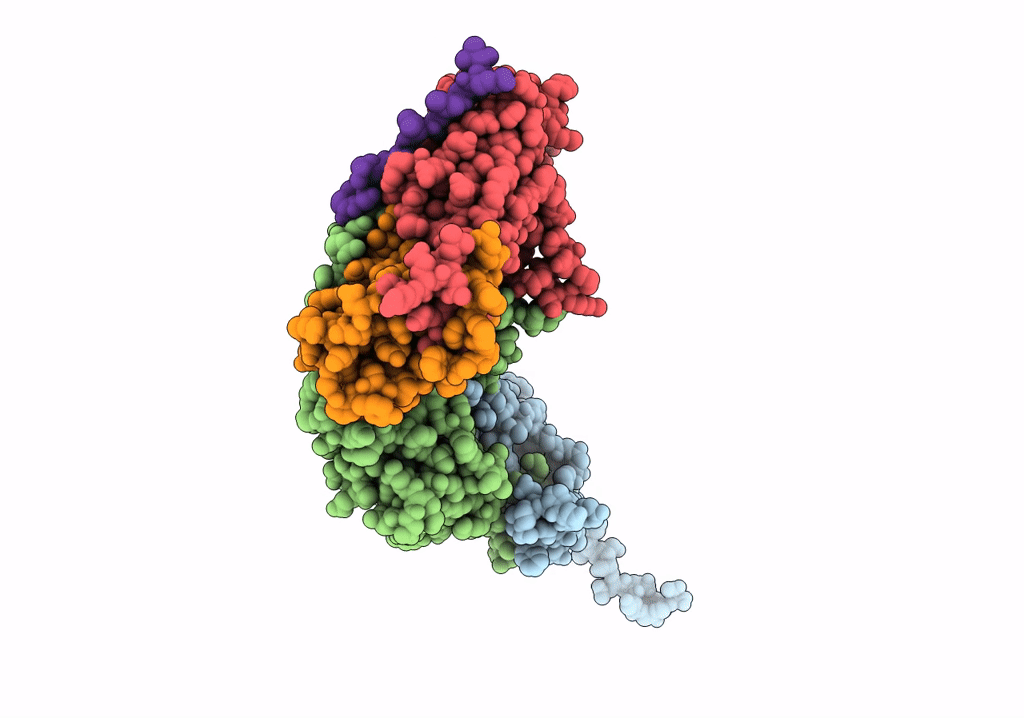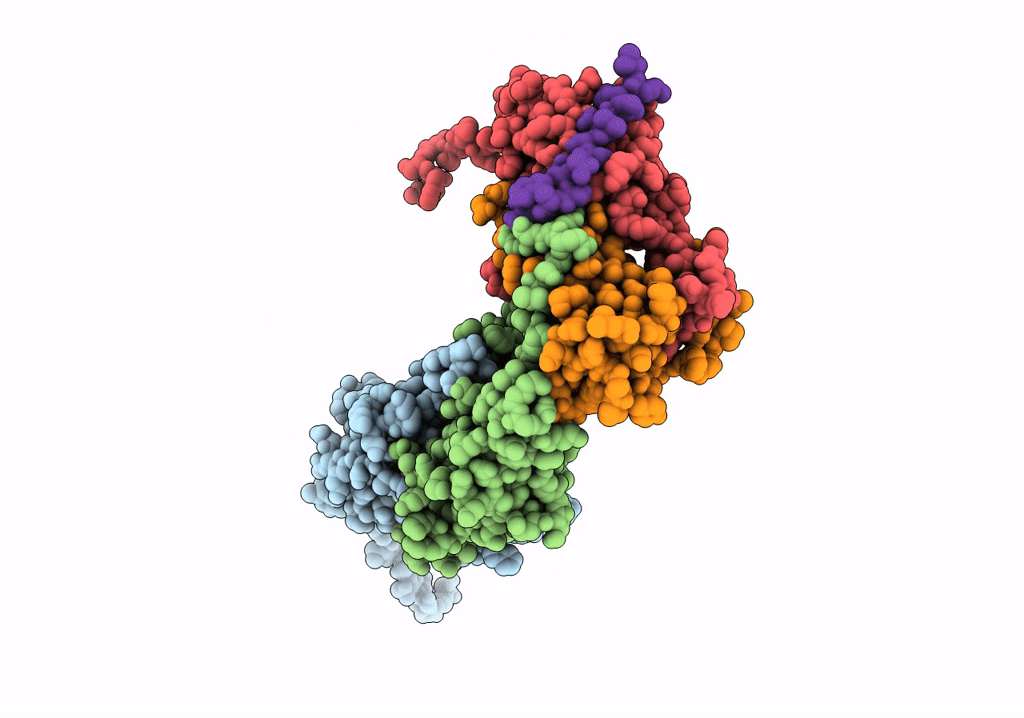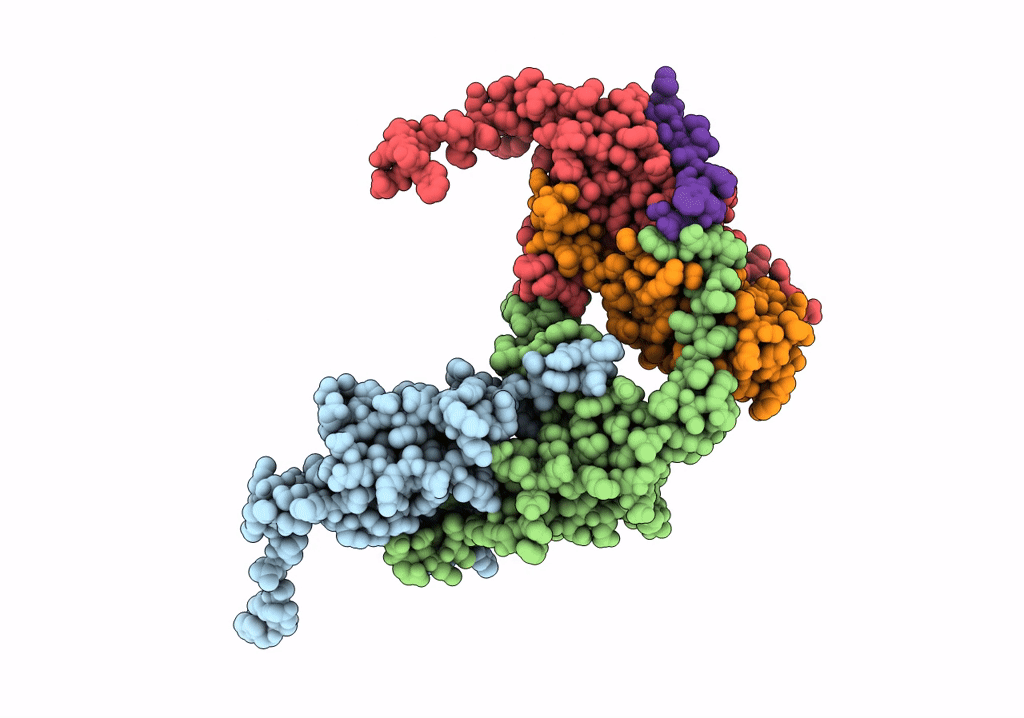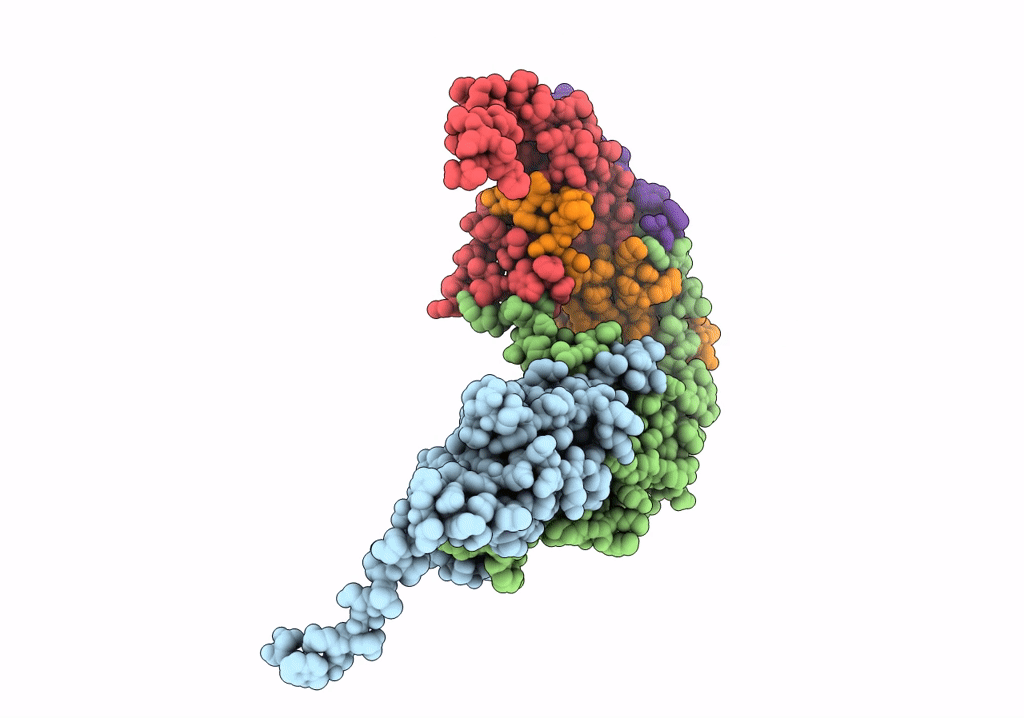
Pseudo-atomic model for Hsp26 residues 63 to 214. Please be advised that the target map is not of sufficient resolution to unambiguously position backbone or side chain atoms. This model represents a likely fit.
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Resolution:
7.80 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


