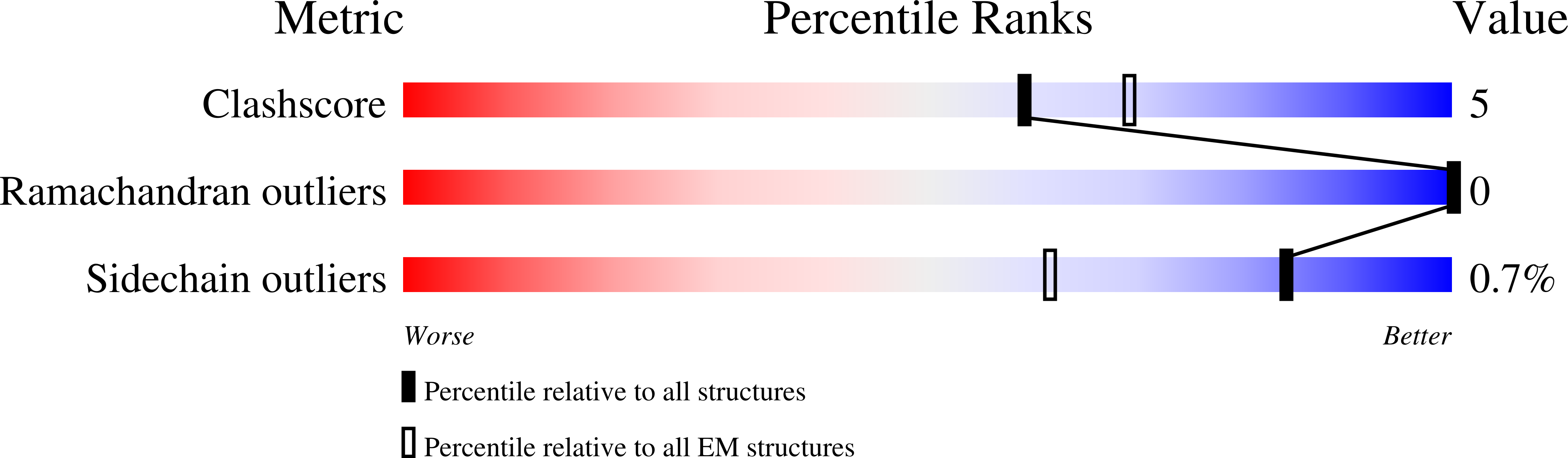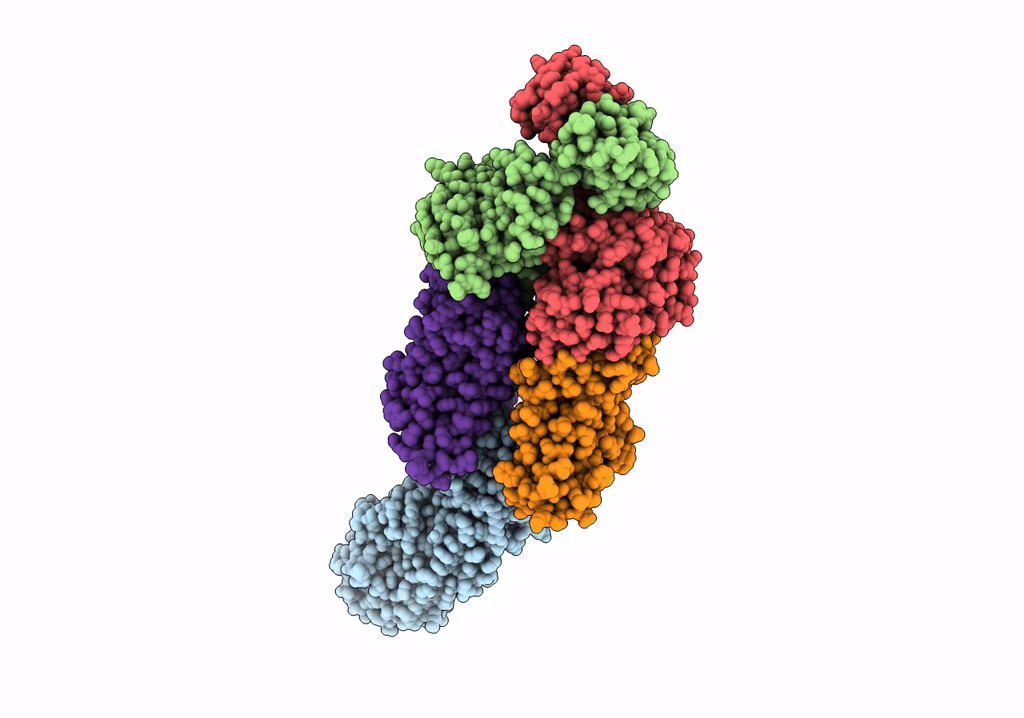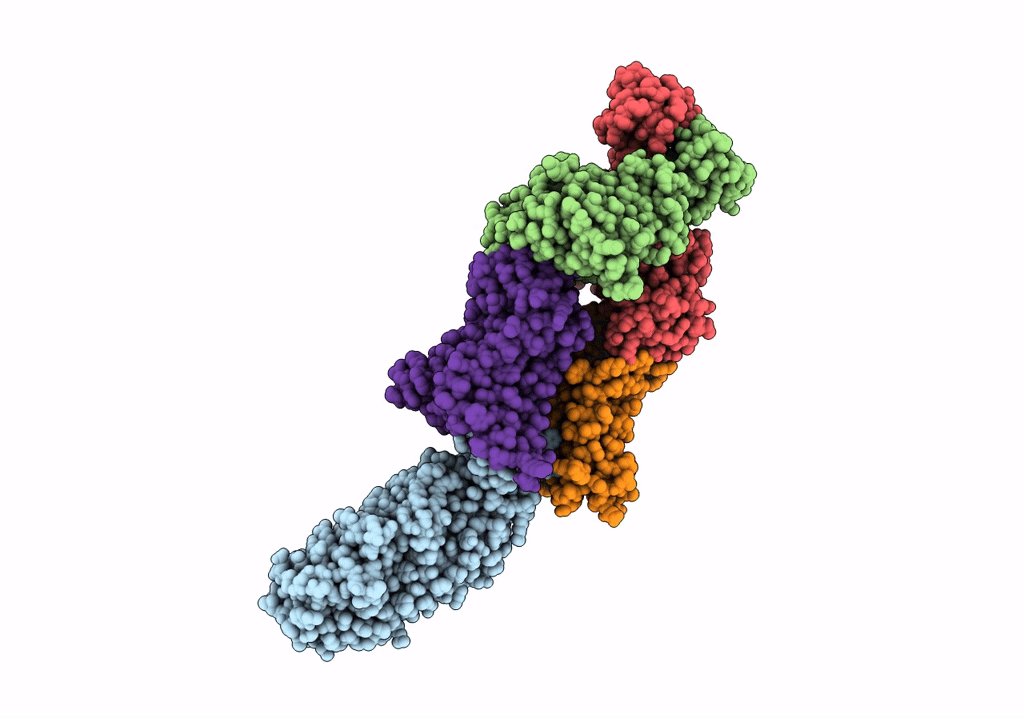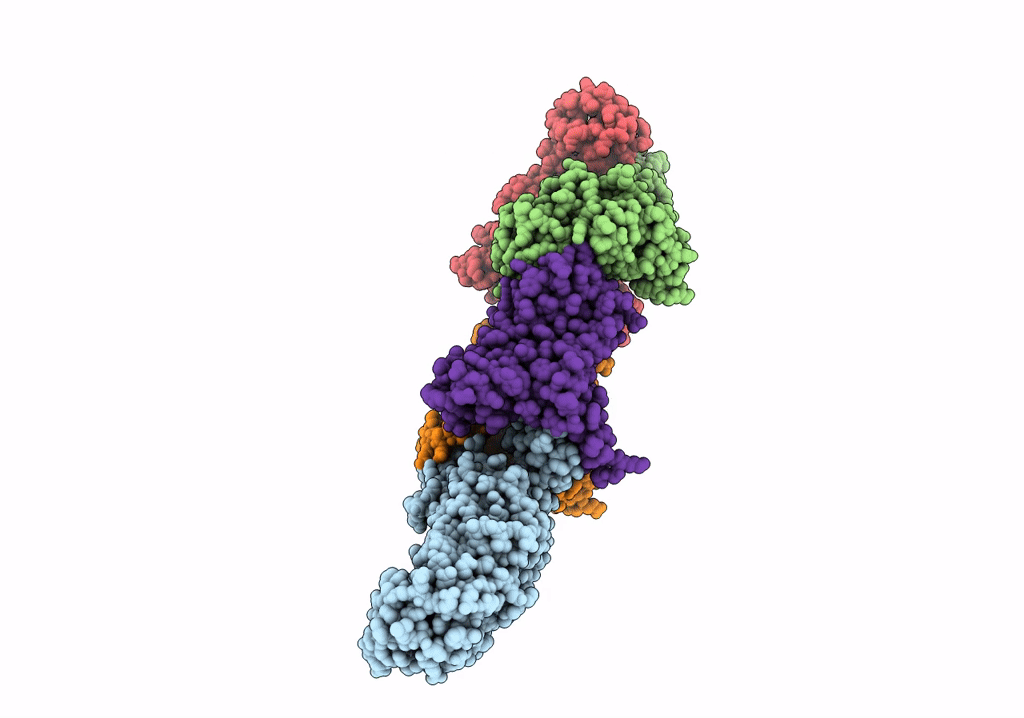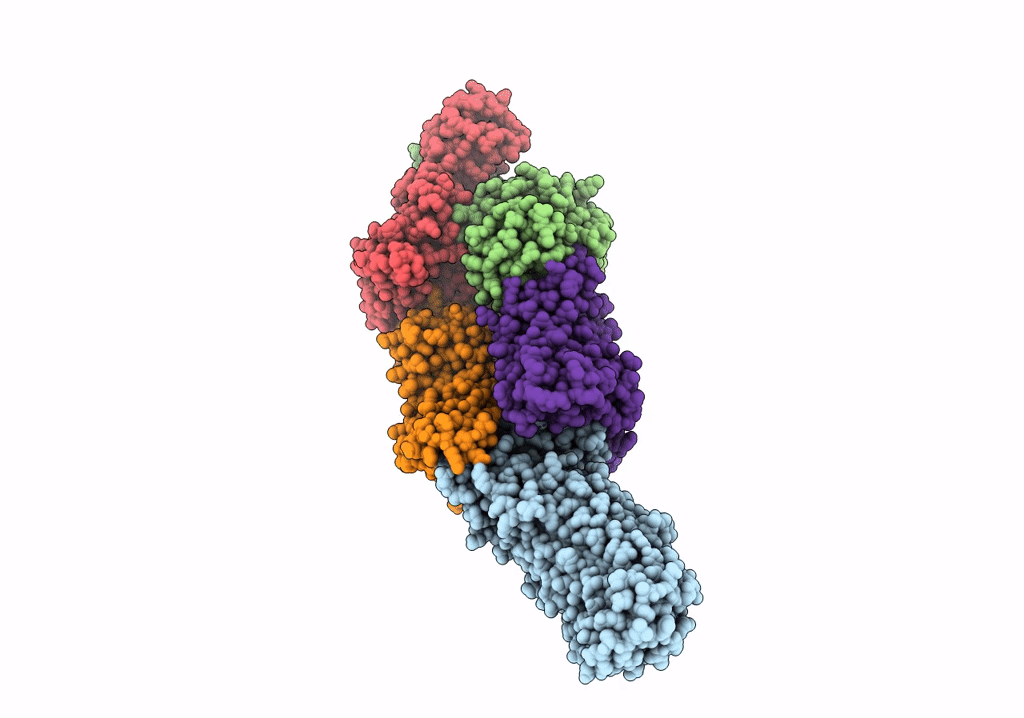
Deposition Date
2021-03-28
Release Date
2022-04-13
Last Version Date
2024-07-10
Entry Detail
PDB ID:
7O10
Keywords:
Title:
ABC transporter NosDFY, nucleotide-free in GDN, R-domain 2
Biological Source:
Source Organism(s):
Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (Taxon ID: 32042)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.60 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE