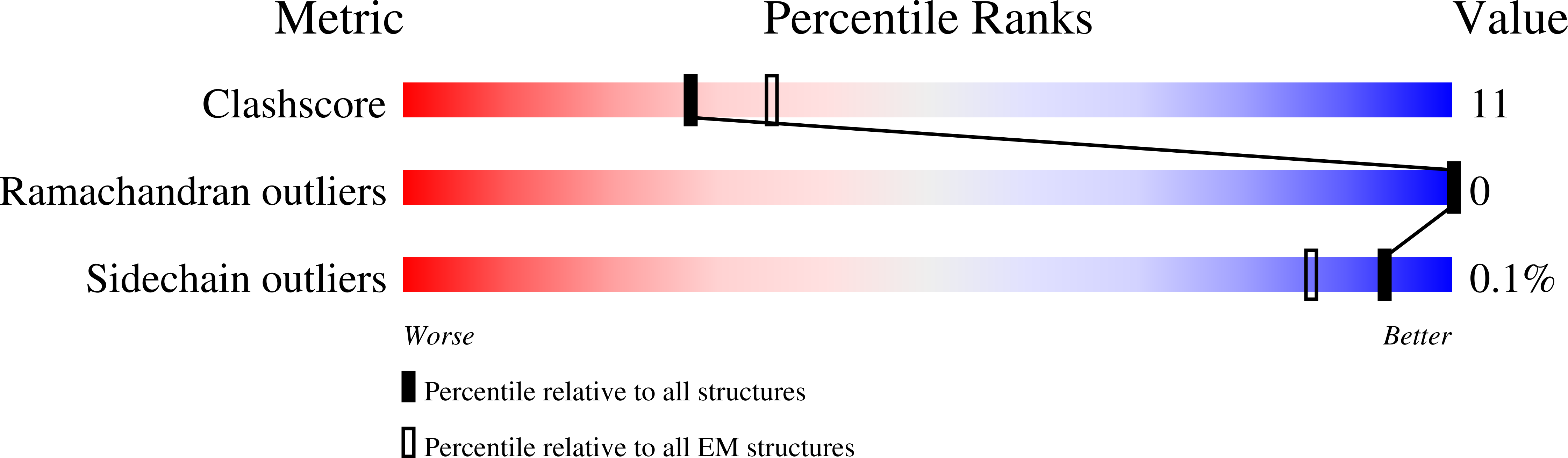
Deposition Date
2021-03-24
Release Date
2021-09-29
Last Version Date
2024-10-23
Entry Detail
PDB ID:
7NZM
Keywords:
Title:
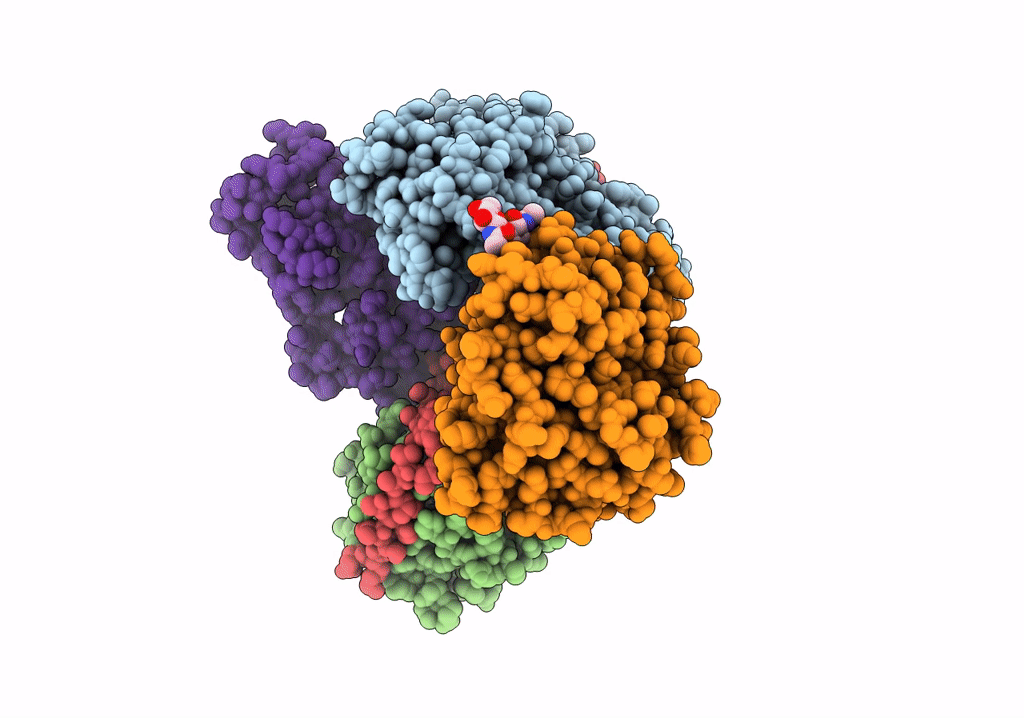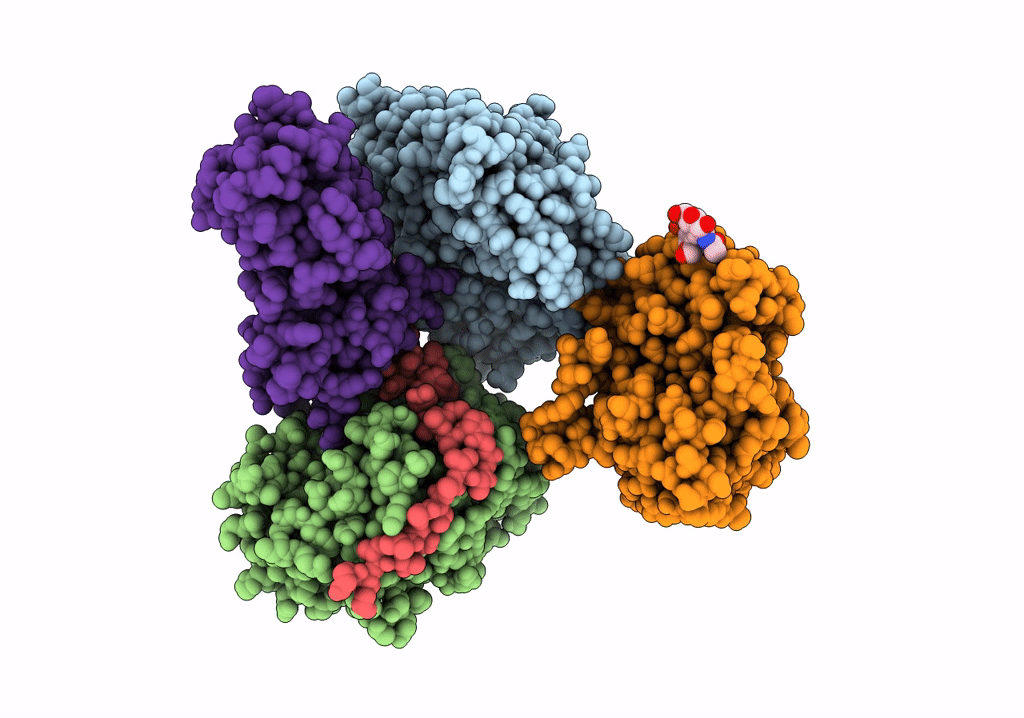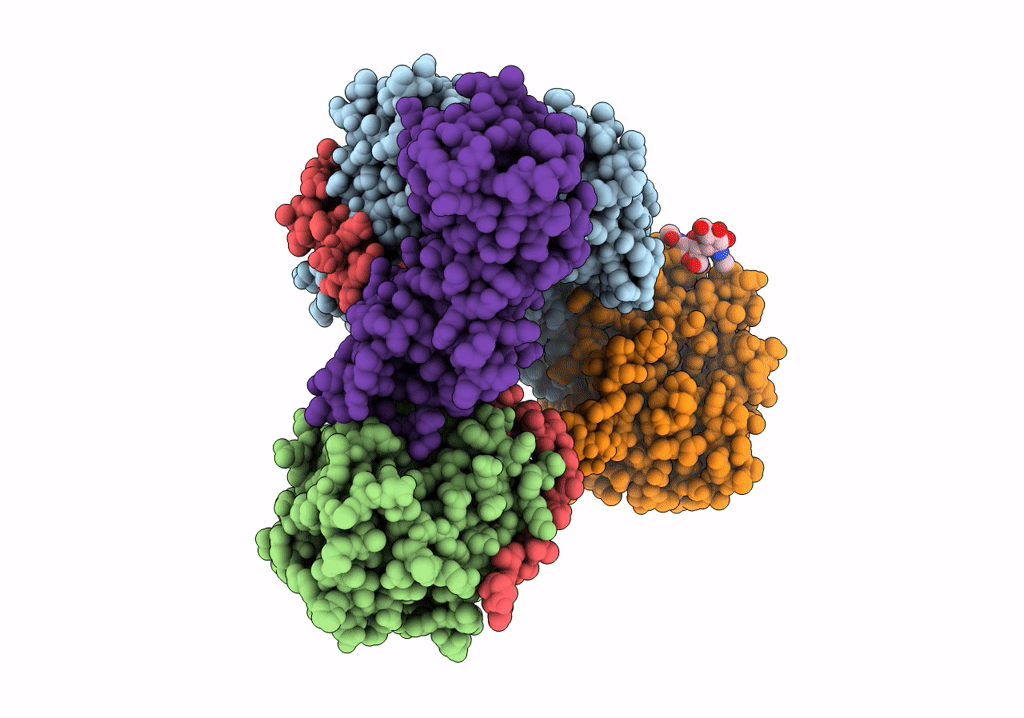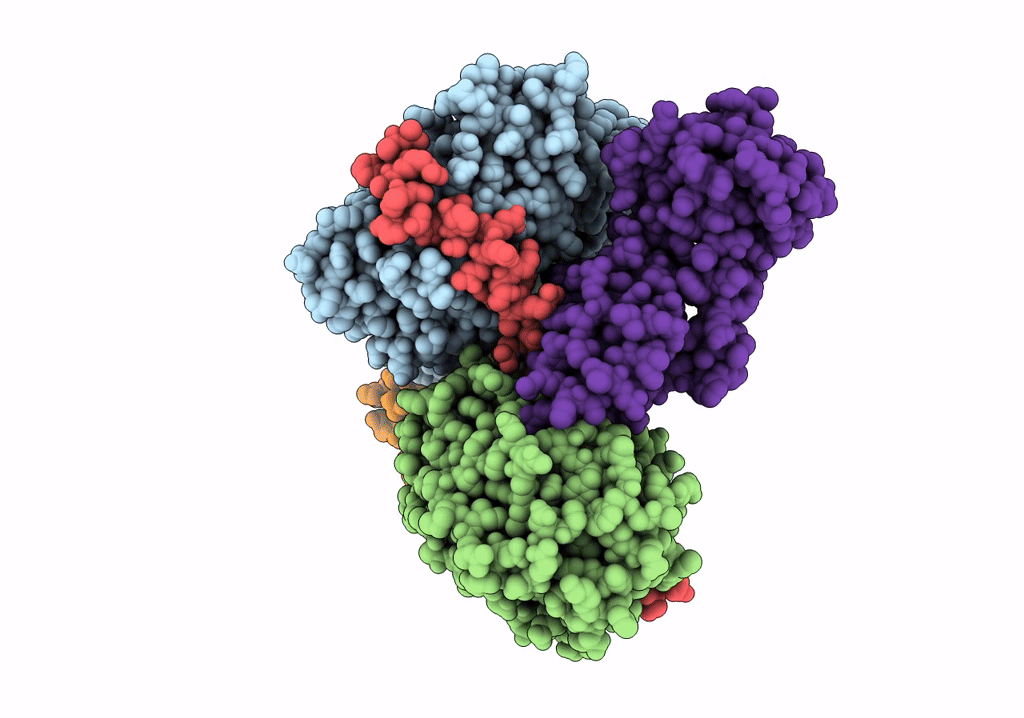
Cryo-EM structure of pre-dephosphorylation complex of phosphorylated eIF2alpha with trapped holophosphatase (PP1A_D64A/PPP1R15A/G-actin/DNase I)
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Oryctolagus cuniculus (Taxon ID: 9986)
Escherichia coli (strain K12) (Taxon ID: 83333)
Bos taurus (Taxon ID: 9913)
Oryctolagus cuniculus (Taxon ID: 9986)
Escherichia coli (strain K12) (Taxon ID: 83333)
Bos taurus (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.96 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE