Deposition Date
2021-03-21
Release Date
2021-11-24
Last Version Date
2024-01-31
Entry Detail
PDB ID:
7NY6
Keywords:
Title:
Crystal structure of the Capsaspora owczarzaki macroH2A macrodomain
Biological Source:
Source Organism:
Capsaspora owczarzaki (strain ATCC 30864) (Taxon ID: 595528)
Host Organism:
Method Details:
Experimental Method:
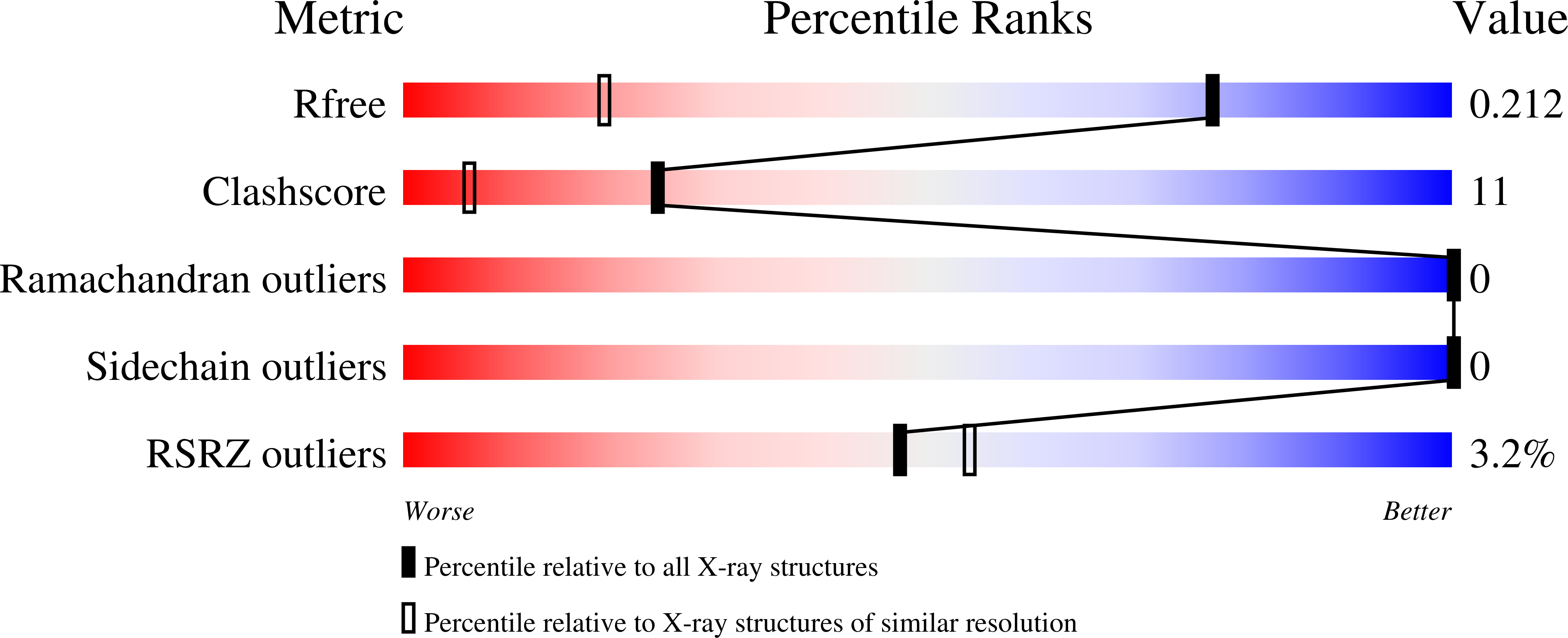
Resolution:
1.34 Å
R-Value Free:
0.21
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 1 21 1