Deposition Date
2021-03-19
Release Date
2021-10-06
Last Version Date
2024-06-19
Entry Detail
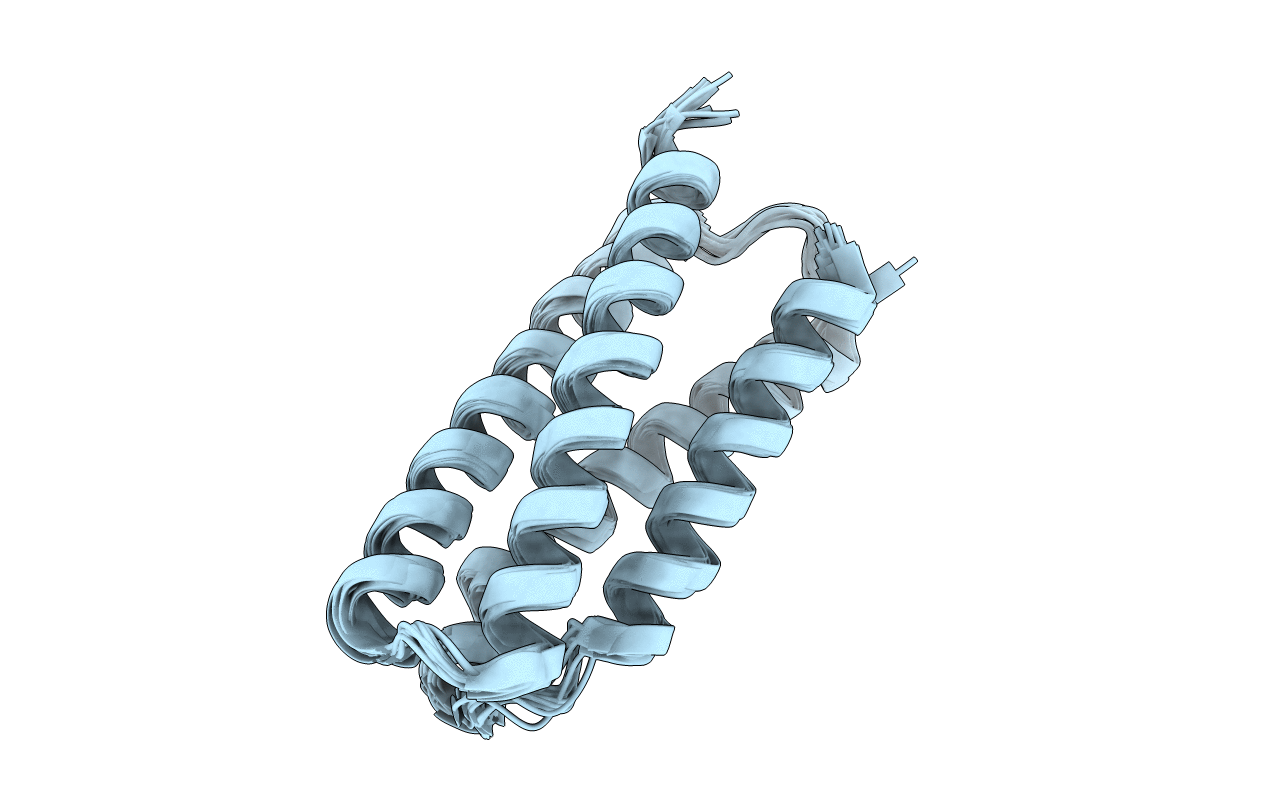
PDB ID:
7NY0
Keywords:
Title:
Solution structure of Boskar4; a de novo designed G-CSF agonist
Biological Source:
Source Organism:
synthetic construct (Taxon ID: 32630)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
10800
Conformers Submitted:
17
Selection Criteria:
back calculated data agree with experimental NOESY spectrum


