Deposition Date
2021-03-09
Release Date
2021-04-28
Last Version Date
2024-10-23
Entry Detail
PDB ID:
7NTC
Keywords:
Title:
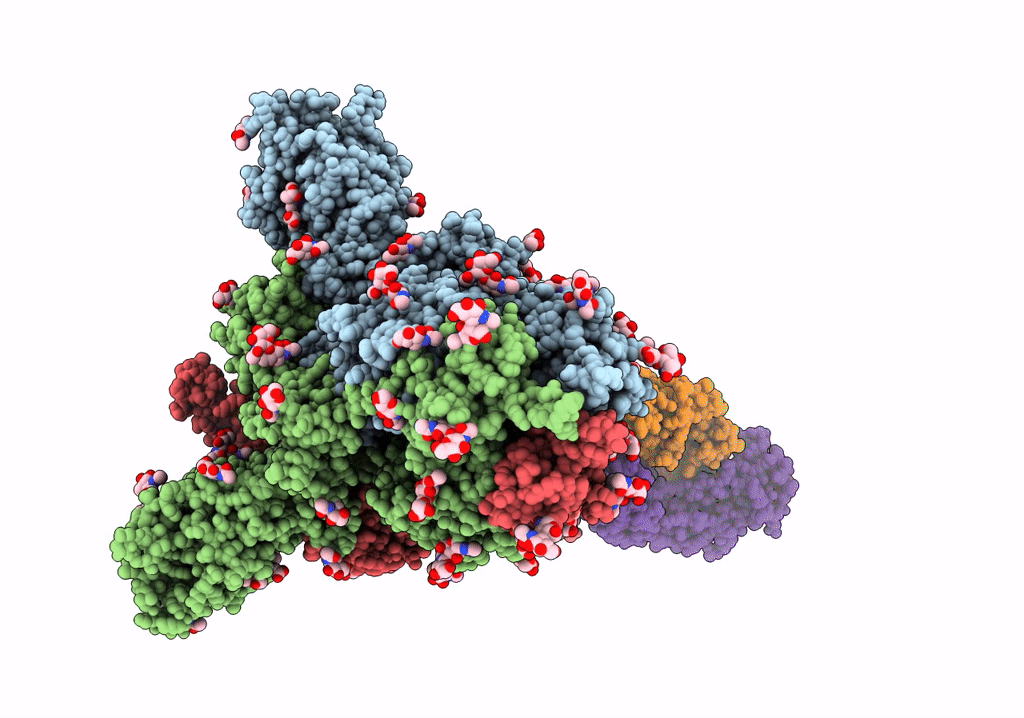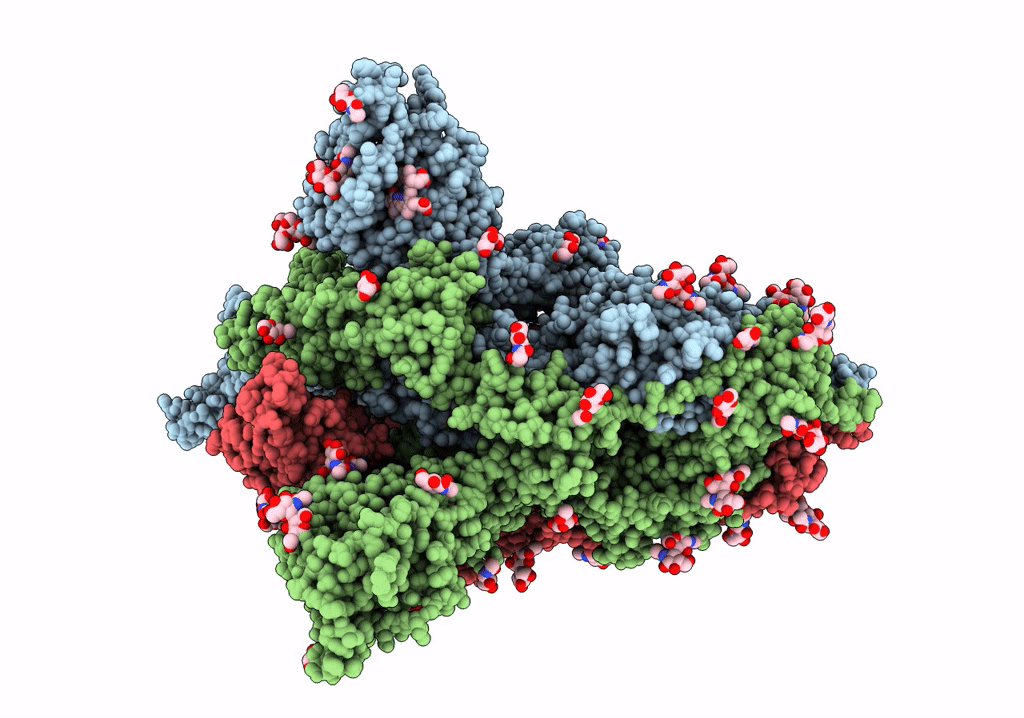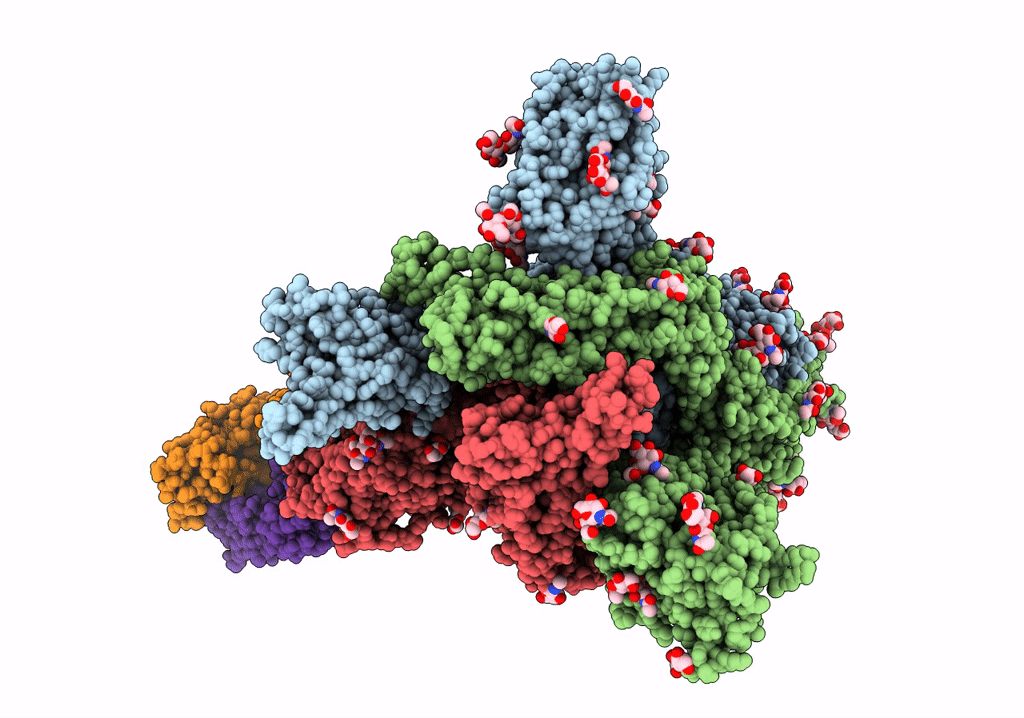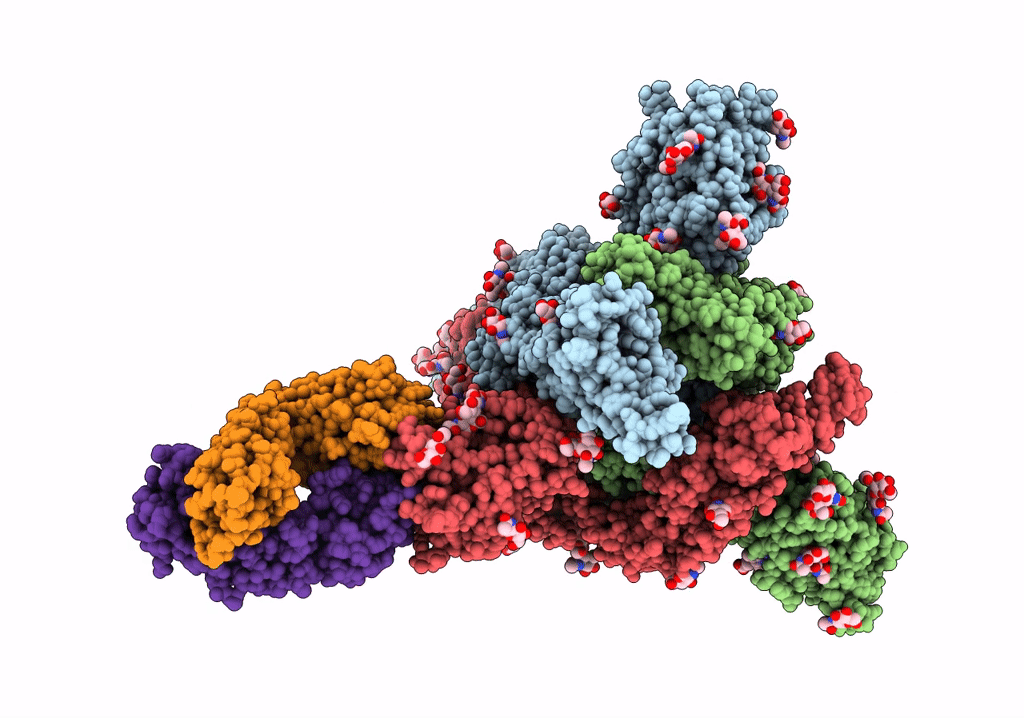
Trimeric SARS-CoV-2 spike ectodomain bound to P008_056 Fab
Biological Source:
Source Organism(s):
Severe acute respiratory syndrome coronavirus 2 (Taxon ID: 2697049)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.60 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


