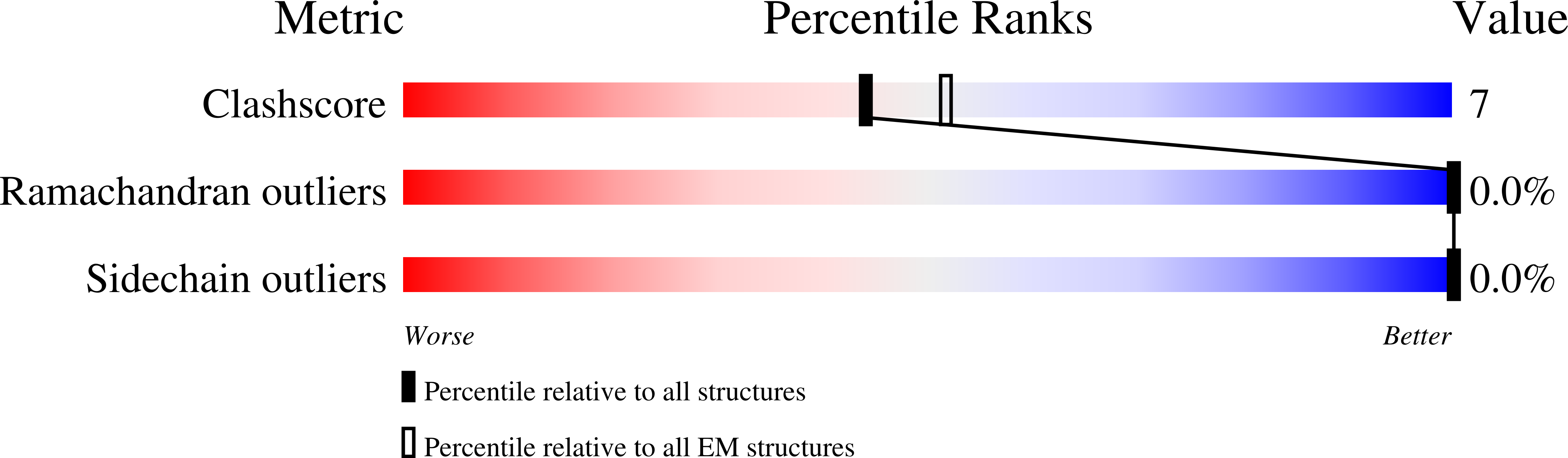
Deposition Date
2021-02-12
Release Date
2021-10-13
Last Version Date
2024-10-23
Entry Detail
PDB ID:
7NIE
Keywords:
Title:
putative glycerol kinase-like proteins anchored on an array of voltage dependent anion channels in the outer mitochondrial membrane of pig sperm mitochondria
Biological Source:
Source Organism(s):
Sus scrofa (Taxon ID: 9823)
Method Details:
Experimental Method:
Resolution:
35.00 Å
Aggregation State:
CELL
Reconstruction Method:
SUBTOMOGRAM AVERAGING