Deposition Date
2021-01-25
Release Date
2021-10-13
Last Version Date
2024-07-10
Entry Detail
PDB ID:
7NBA
Keywords:
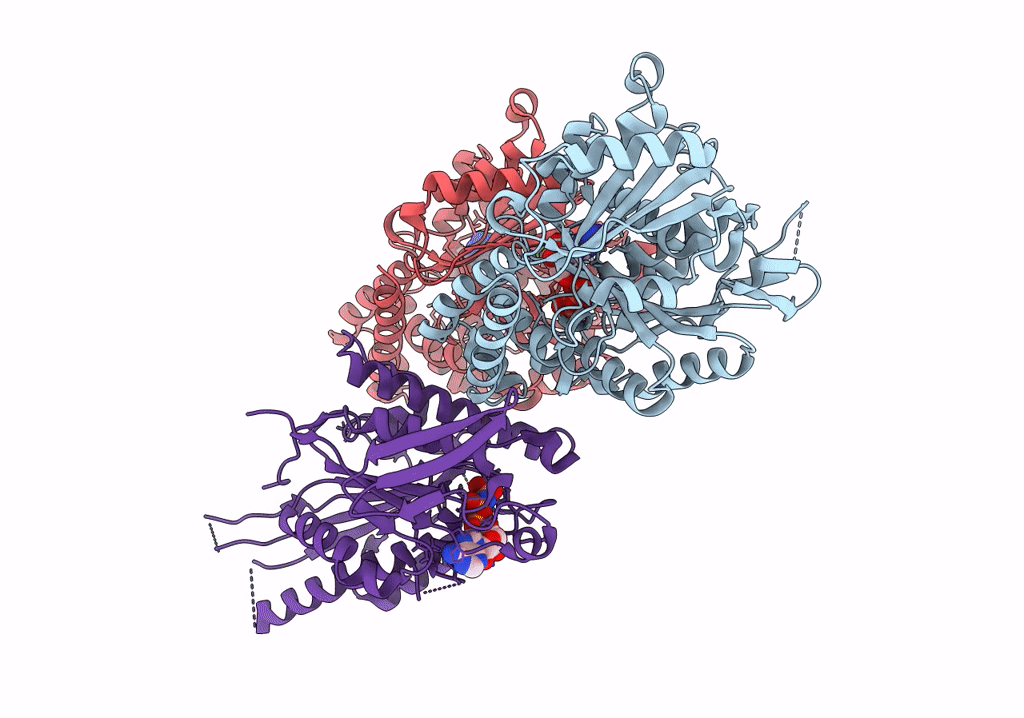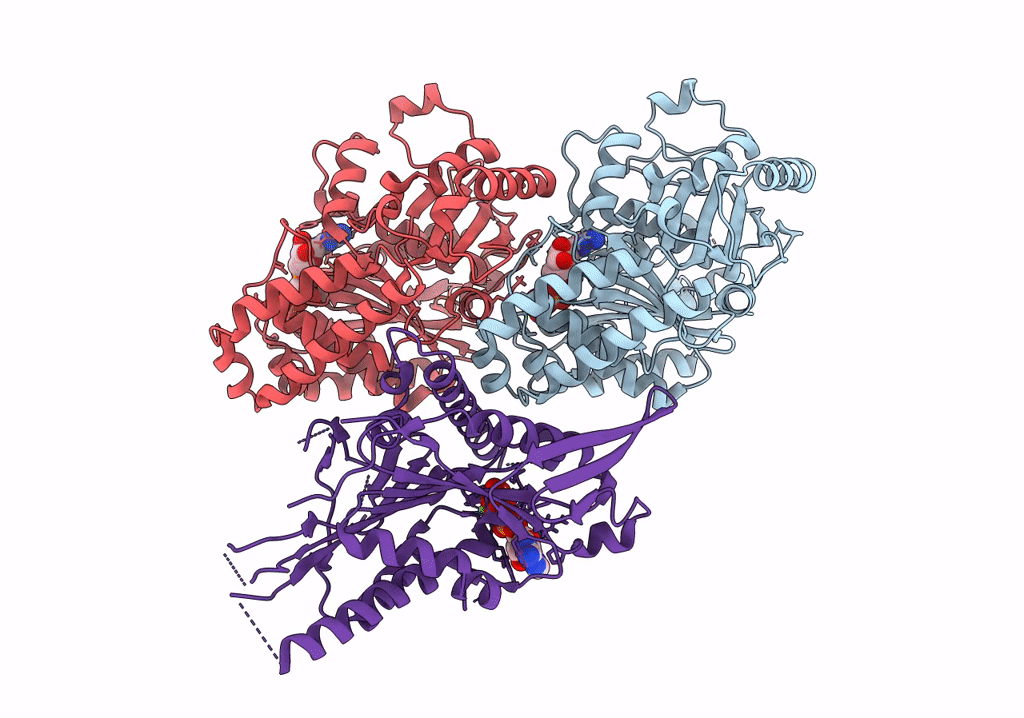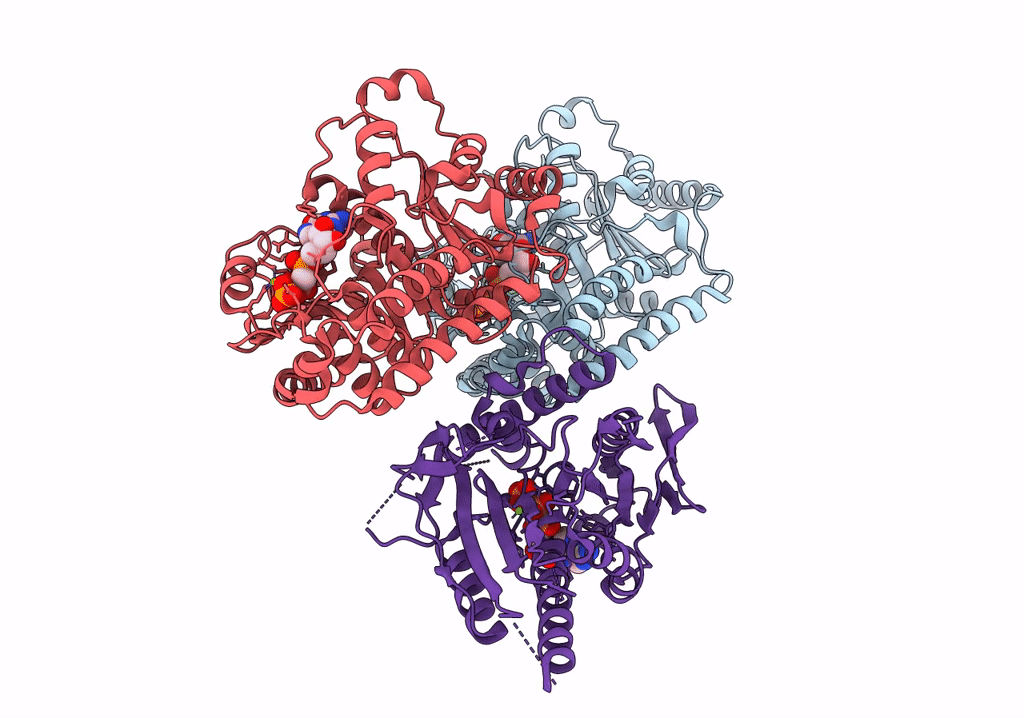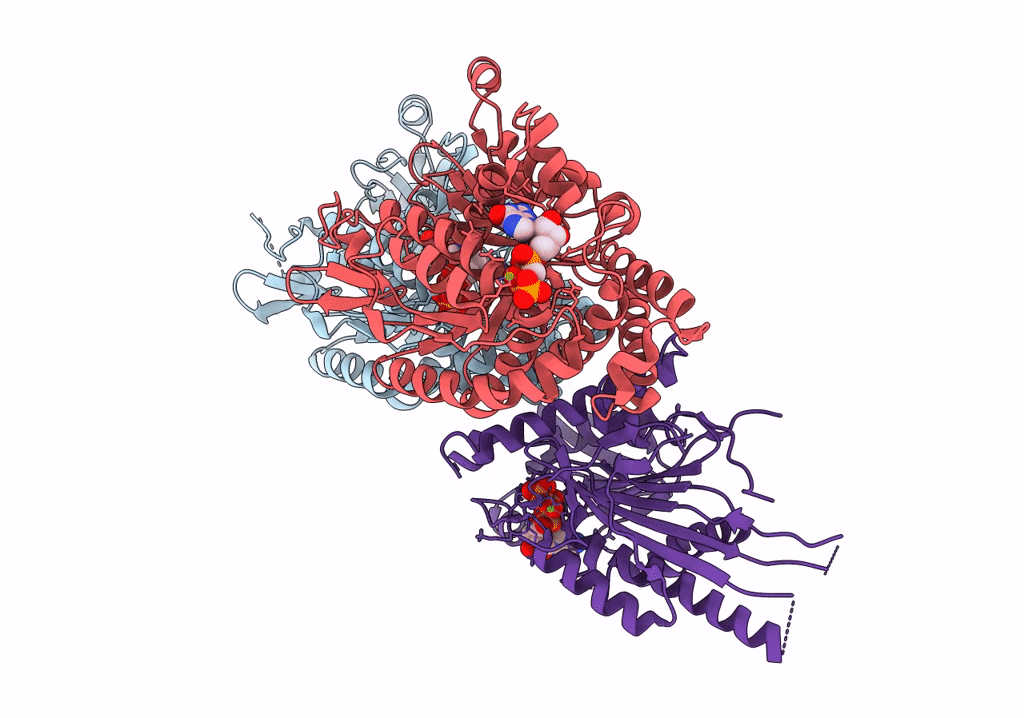
Title:
Plasmodium falciparum kinesin-5 motor domain bound to AMPPNP, complexed with 14 protofilament microtubule.
Biological Source:
Source Organism(s):
Plasmodium falciparum (isolate NF54) (Taxon ID: 5843)
Sus scrofa (Taxon ID: 9823)
Sus scrofa (Taxon ID: 9823)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.00 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL