Deposition Date
2021-06-15
Release Date
2022-04-06
Last Version Date
2023-11-15
Entry Detail
PDB ID:
7N8Q
Keywords:
Title:
Rhesusized RV305 DH677.3 Fab bound to Clade A/E 93TH057 HIV-1 gp120 core.
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Macaca mulatta (Taxon ID: 9544)
synthetic construct (Taxon ID: 32630)
Macaca mulatta (Taxon ID: 9544)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
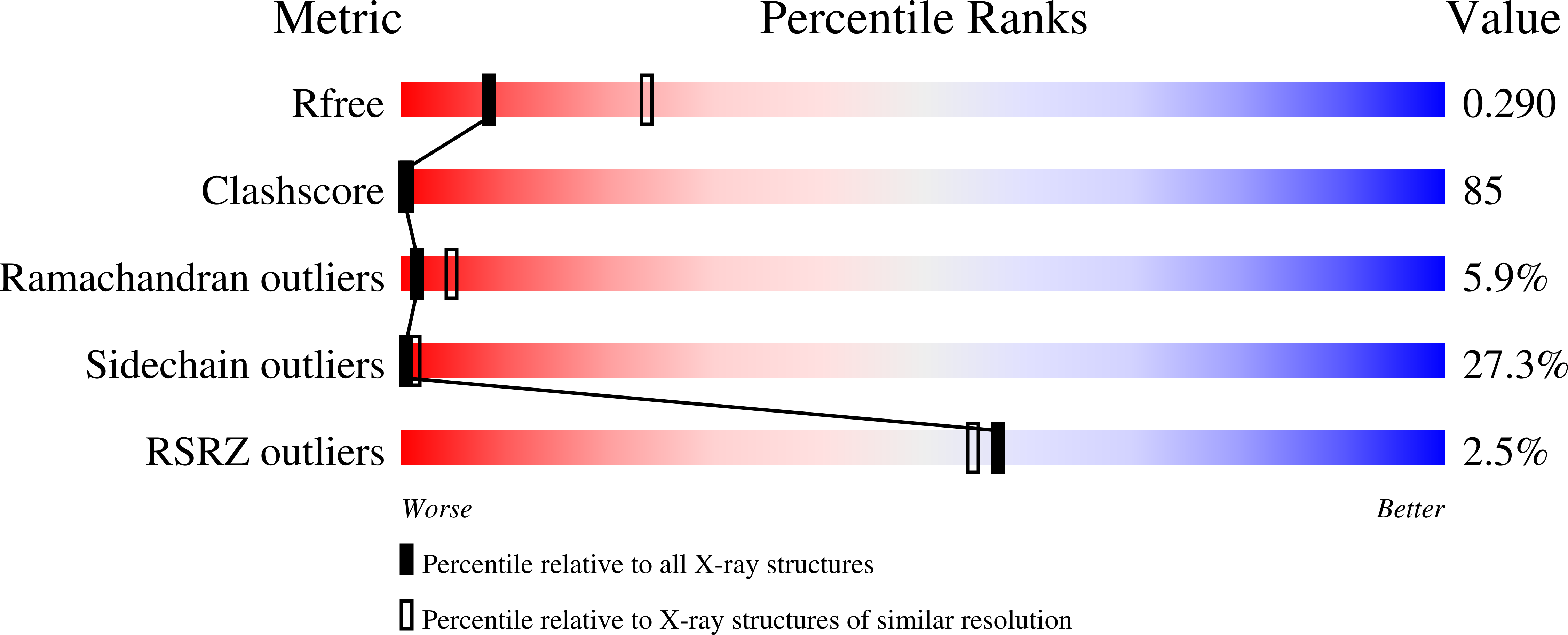
Resolution:
2.90 Å
R-Value Free:
0.29
R-Value Work:
0.25
R-Value Observed:
0.25
Space Group:
P 1 21 1