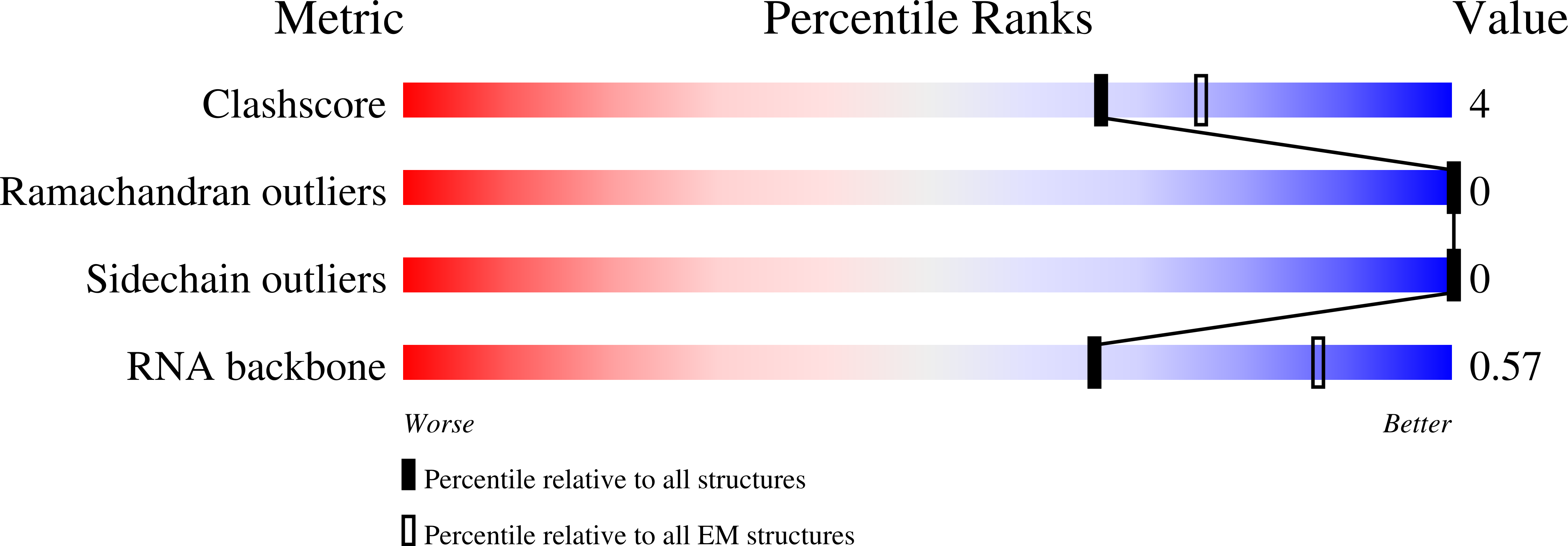Deposition Date
2021-05-25
Release Date
2021-07-28
Last Version Date
2025-05-28
Entry Detail
PDB ID:
7N0D
Keywords:
Title:
Cryo-EM structure of the tetrameric form of SARS-CoV-2 nsp10-nsp14 (E191A)-RNA complex
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE